Abstract
Background. Total skin electron beam therapy (TSEBT) is an effective palliative treatment for cutaneous T-cell lymphoma (CTCL). In the present study we reviewed the clinical response to TSEBT in Danish patients with CTCL. Material and methods. This retrospective study included 35 patients with CTCL treated with TSEBT in Denmark from 2001 to 2008 and followed for a median time of 7.6 months (range 3 days–3.7 years). Twenty five patients were treated with high-dose (30 Gy) and 10 patients in a protocol with low-dose (4 Gy) TSEBT. Results. Patients treated with low-dose therapy had inadequate response to treatment compared to patients treated with high-dose. Consequently the study with low-dose was discontinued and published. In patients treated with high-dose the overall response rate was 100%. Complete response (CR) rate was 68% and CR occurred after a median time of 2.1 months (range 1.8 months–2.0 years). We found no difference in CR rate in patients with T2 (66.7%) and T3 disease (78.6%) (p = 0.64). Following CR 82.4% relapsed at a median time of four months (range 12 days–11.5 months). Relapse-free-survival was similar in patients with T2 and T3 disease (p = 0.77). Progressive disease (PD) was experienced in 28.0% and the median time to PD was 9.0 months (range 4.6–44.3 months). Overall progression-free survival was 95.3%, 72.1% and 64.1% after 0.5-, 1- and 2-years. Effects of initial therapy on TSEBT treatment response and side effects to TSEBT were also analyzed. Conclusion. In conclusion, the present study confirms that high-dose TSEBT is an effective, but generally not a curative therapy in the management of CTCL. High-dose treatment yielded significantly better results than low-dose treatment with 4 Gy. TSEBT offers significant palliation in most patients when other skin-directed or systemic treatments have failed.
Cutaneous T-cell lymphoma (CTCL) is characterized by malignant proliferation of skin-homing T-helper cells within the outer layer of the epidermis and dermis [Citation1,Citation2]. The most common subgroup of CTCL is mycosis fungoides (MF) [Citation1,Citation2]. The etiology of CTCL is unknown, but genetic, infective and environmental causes have been suggested, although none has been substantiated [Citation1]. The incidence is approximately three patients per million population per year and increases with advancing age [Citation3]. Males are 2.2 times as likely to develop CTCL as females [Citation4]. The initial course of patients with CTCL is usually relatively indolent, but most patients develop an orderly progression from limited patches to more generalized patches, plaques, tumours and finally, nodal or visceral involvement [Citation4]. Patients with CTCL are classified according to a clinical staging system based on the extent of skin involvement (T-stage), presence of lymph node and visceral involvement (TNM-classification system), which has prognostic validity [Citation3].
Treatment of patients with CTCL includes both topical and systemic therapies [Citation5]. The most common therapies include psoralene plus UVA irradiation (PUVA), total skin electron beam therapy (TSEBT) and topical- and systemic chemotherapy [Citation4]. These interventions are rarely curative, partial responses, progression and relapses do often occur and prolonged disease-free survival is rare except in patients with early stage CTCL [Citation6]. TSEBT has been used in treatment of CTCL since 1951 and represents an effective treatment in early stages of MF and an effective, but palliative treatment in more advanced stages [Citation5,Citation6]. It has minimal penetration to dermis and deeper tissues and therefore causes relatively few side effects. TSEBT should be considered as initial therapy for patients with thickened plaques, because TSEBT is more effective in the depth of the plaques than topical therapies, such as nitrogen mustard and phototherapy [Citation5]. In patients with rapid progression of disease and patients, experiencing failure of local therapy TSEBT can be an effective treatment for achieving disease control [Citation5]. The total doses applied to the skin are usually 30–36 Gy over 8–10 weeks [Citation7]. The overall clinical response rates after TSEBT are nearly 100% and complete response rates range from 98% for limited plaque stage to 40% for tumor stage [Citation5]. However, the majority of the patients treated with TSEBT will experience recurrent disease [Citation8]. To delay the time to relapse, maintenance and adjuvant therapies are often used after TSEBT.
Previous studies reporting the therapeutic effects of TSEBT have mainly focused on the response related to specific stages of CTCL, effects of adjuvant therapies following TSEBT, and doses and energies of TSEBT related to treatment outcome and side effects. Previously we have reported from a prospective, open-label study of low-dose (4 Gy) TSEBT in MF. Only one patient did not respond to TSEBT, but nine of 10 patients relapsed within an average of 2.4 months. Thus, the long-term disease control was poor and consequently the study was discontinued and published [Citation8]. In the present study we examined the clinical response and side effects to TSEBT in all stages of CTCL to report updated results of effects of TSEBT in Denmark from 2001 to 2008. Furthermore, we report the impact of previous therapies on clinical response to TSEBT and the effect of clinical status before initiation of TSEBT.
Material and methods
Included in this retrospective study were patients with CTCL, who initiated treatment with TSEBT in Denmark between January 2001 and November 2008, a total of 35 patients. All patients underwent a complete physical examination consisting of grading of the extent of skin involvement and careful palpation of all the major lymph node areas. Patients with clinical lymphadenopathy underwent lymph node biopsy or extirpation. Complete blood samples, including examination for Sézary cells and computed tomography (CT) examination were obtained in most patients. Subsequently patients with CTCL were staged according to the ISCL/EORTC staging system at time of diagnosis, at time of referral to TSEBT and at the following clinical examinations.
Previous treatments until TSEBT referral and treatment response were registered. Date of complete response, relapse, disease progression and side effects of TSEBT were registered until the patients died or terminated clinical control. More detailed follow-up data, including clinical characteristics, stage, therapeutic response and side effects, were registered after 3 months, 1-, 2- and 3-years. All data were registered in medical journals and archived in a database.
Clinical response to treatment was determined by physical examination. For the purpose of these analyses, complete response (CR) was defined as complete clinical regression of all skin lesions, partial response (PR) as any response less than complete but greater than 50% clinical improvement and no response (NR) divided in stable disease (SD) and progressive disease (PD). PD was defined as worsening of the disease to a higher T classification or a higher clinical stage in patients with CTCL.
TSEB
TSEB was administered using a technique similar to the technique originally developed at Standford University [Citation9,Citation10]. A 6 MeV six dual field technique was used, with the patient standing in six angular orientations about a vertical axis so the entire body surface was exposed to the beam. The positions were divided into a two-day cycle. On day 1 the patient was treated with a perpendicular anterior field and two oblique posterior fields and on day 2 a perpendicular posterior field and two oblique anterior fields.
For each of the six positions two gantry angles were used. The two gantry angles, 90° ± 19°, were chosen so that the central axis would pass above and under the patient thus avoiding the x-ray fluence of a forward directed beam. The field size was set to 40 cm × 40 cm, the dose rate was 888 MU/min (at a distance of 1.6 m), which corresponds to 1.3 Gy/min in the center of the patient plane. The treatment of one field would take approximately 30 s.
In fields where the patient’s face was turned towards the accelerator eye shielding consisting of 3 mm lead was used (except in patients who had been operated for cataract). Starting about midway through the treatment, the toes and fingers were shielded with 3 mm lead to avoid overdosing.
The treatment was given with a source to skin distance of 370 cm with the patient standing about 20 cm behind a 0.5 cm thick, 1 × 2 m2 acrylic panel. The panel works as an energy degrader, which means that the depth dose falls off closer to the body surface yielding a better dose in the most superficial parts of the skin. The panel also improves the dose uniformity, especially on oblique surfaces [Citation11].
Supplemental treatment was given to portions of the body surface that were shadowed and received relatively lower doses, such as the scalp, the perineum, the soles of the feet, and areas underneath the breasts or other skin folds. Typically, 6 MeV electron therapy with 1 cm of bolus was given to these areas. Patients with thick (≥ 1½–2 cm) tumors were treated with local electron fields, usually 15 Gy in 5 fractions, before start of TSEBT. However, most of the patients with T3 disease did not have lesions thicker than 1–1½ cm. In this situation, in our experience, the tumors often shrunk during treatment and became so thin at the end of treatment that a complete remission was obtained.
Patients treated with high-dose TSEBT received a total dose of 30 Gy with 20 Gy as supplemental treatment to shadowed areas. Patients treated with low-dose TSEBT received a total dose of 4 Gy with 4 Gy as supplemental treatment to shadowed areas.
Statistics
Event curves for CR, relapse and PD were drawn using the Kaplan-Meier approach and compared by log rank tests. Confidence intervals (CI) for proportions were calculated using Jeffry's method and equality of proportions assessed by Fisher's exact test with Cornfields CIs for the corresponding odds ratios (ORs). Considered significance level is 5%.
Results
Demographics and disease characteristics
Median age at time of TSEBT referral was 66 years (range 41–85 years). Female-male ratio was 1:1.9. All patients were diagnosed with CTCL, mainly MF (). Median time from confirmed diagnosis to TSEBT referral was 14.4 months (range 0 days–28 years). At time of referral the majority of the patients had T2 and T3 disease (). Almost all patients (97.1%) were treated with other therapies prior to TSEBT, 94.1% received topical therapies including topical corticosteroids, 8-methoxypsoralen and ultraviolet light (PUVA), local radiotherapy, nitrogen mustard and narrow-band-UVB, and 77.1% received systemic therapies. The most commonly used systemic therapies were prednisolone, methotrexate and acitretin (data not shown). At time of TSEBT referral the treatment response was PR in 7.7%, SD in 53.8% and PD in 33.3% patients. Median time of follow-up was 7.6 months (range 3 days–3.7 years) and median time from referral to initiation of TSEBT was 2.1 months (range 28 days–4.8 months). TSEBT was divided into two groups, high-dose therapy (26–30 Gy, 1 Gy per fraction) given in 73.7% (25 patients) of the patients and low-dose therapy (4 Gy, 1 Gy per fraction) given in 26.3% (10 patients) of the patients, the latter in a protocol which has previously been published [Citation8]. Patients treated with high-dose therapy were in stage T2–T4 at time of referral and patients treated with low-dose were in stage T1–T3 (). In addition boosts (20 Gy in 10 fractions for high-dose patients, 4 Gy in two fractions for low-dose patients) were given to the scalp, eyelids, perineum and feet in 60.0%, 37.1%, 68.6% and 60.0%, respectively, of the patients. Median treatment duration was 58 days (range 50–102 days) in patients treated with high-dose and four days (range 3–4 days) in patients treated with low-dose therapy. Two patients had an unintended break during their course of therapy. One patient died from chronic heart failure during TSEBT.
Table I. Diagnosis (A) and clinical characteristics (B) at time of referral to TSEBT.
Response to TSEBT
Patients treated with high-dose TSEBT had a higher CR rate than patients treated with low-dose TSEBT, 68.0% (CI, 48.5–83.6%) and 10.0% (CI, 1.1–38.1%), respectively, (p < 0.01). CR occurred after a median time of 2.1 months (range 1.8 months–2.0 years) in high-dose and 11.5 months in low-dose TSEBT. PD rates were similar in patients receiving high- (28.0%) (CI, 13.5–47.3%) and low-dose (40.0%) (CI, 15.3–69.6%) TSEBT (p = 0.69). Patients receiving high-dose TSEBT had a higher freedom-from-progression (FFP) than patients receiving low-dose TSEBT (p < 0.01) (). Thus, patients treated with low-dose therapy had a clearly inadequate response to treatment compared to patients treated with the standard dose. Consequently the study with low-dose treatment was discontinued and published. The following results are therefore only from patients treated with high-dose therapy. P-values above are uncorrected for the partly data driven discontinuation of the low-dose study of which however we suspect no conclusive influence.
Figure 1. Freedom-from-progression (FFP) was significantly increased in patients treated with high-dose (solid line) (n = 25) compared to low-dose (stabled line) (n = 10) TSEBT treated patients, p < 0.01.
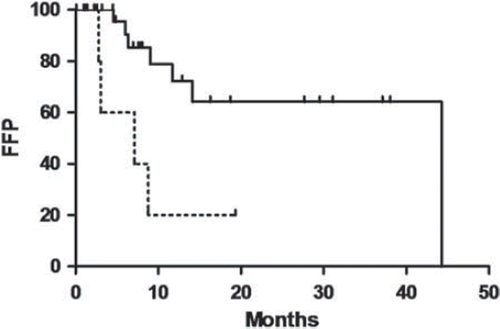
Overall response rate (CR + PR) was 100% (CI, 0.91–100%) (25 patients) (). CR was achieved in 68.0% (CI, 48.5–83.6%). CR was not observed in patients with T4 disease, but in 66.7% (CI, 34.8–89.6%) of patients with T2 and 78.6% (CI, 53.1–93.6%) of patients with T3 disease (). The observed OR of CR of 1.83 (CI, 0.31–10.9%) between the latter two appears somewhat counterintuitive. However as seen by the CI, we find no significant discrepancy with Jones et al. [Citation12] estimating a logistic regression OR of CR for stage T4 against T1 of 0.17 corresponding to an OR for T3 against T2 of 0.55.
Table II. Overall response to high-dose TSEBT.
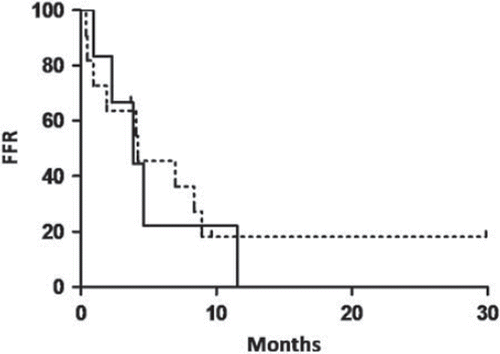
Median time to CR was 2.1 months (range 1.8 months–1.2 years), with no difference in comparing patients with T2 and T3 disease (p = 0.62). Following CR 82.4% (CI, 60–94.8%) relapsed at a median time of 4.0 months (range 12 days–11.5 months). Rates of relapse were similar in patients with T2 (83.3% (CI, 44.2–98.1%) and T3 (81.8% (CI, 53.3–96.0%)) disease (p = 1). We found no differences in freedom-from-relapse (FFR) in patients with T2 and T3 disease (p = 0.77) (). Overall FFR rates after 2-, 6- and 12-months were 70.6%, 38.8% and 9.7%, in patients with T2 disease 83.3%, 22.2% and 0% and in patients with T3 disease 63.6%, 45.5% and 18.2% (). During follow-up one patient with T2 and two patients with T3 disease did not relapse after CR. These patients were followed for four months, 9.6 months and 2.5 years, respectively.
At clinical follow-up three months after TSEBT, response rates (CR + PR) between patients with T2 (88.9% (CI, 58.6–98.8%)) and T3 disease (78.6% (CI, 53.1–93.6%)) did not differ significantly (p = 1). Likewise we found no statistical significant difference between patients with T2 and T3 disease at 1-year follow-up, 71.4% (CI, 35.2–93.5%) and 66.7% (CI, 38.8–87.5%) (p = 1), respectively and 2-years follow-up, 100% (CI, 33.3–100%) and 80.0% (CI, 37.1–97.7%) (p = 1), respectively (data not shown).
Figure 2. Freedom-from-relapse (FFR) outcome of patients treated with high-dose TSEBT with T2 (solid line) and T3 (stabled line) disease, who achieved CR (p = 0.77).
CR rate was slightly higher in patients younger than 66 years (median age) (83.3% (CI, 56.4–96.4%) compared to patients older than 66 years (53.8%) (CI, 28.3–77.9%) (p = 0.20). No association was found between age above or under 66 years and time to CR (p = 0.61), rate of relapse following CR (p = 1) or FFR (p = 0.67). Response to TSEBT treatment was analyzed with regard to gender and we found no difference between the females and males.
Patients with PD at the time of TSEBT referral had a similar CR rate (72.7% (CI, 43.5–91.7%)) as patients with SD (69.2% (CI, 42.3–88.6%) (p = 1). Likewise, time to CR was similar in patients with PD compared to patients with SD at time of referral (p = 0.56). Relapse rate following CR (p = 1) and FFR (p = 0.96) was similar in patients with PD and SD at time of TSEBT referral.
Disease progression
Overall 28.0% (CI, 13.5–47.3%) experienced disease progression, and divided according to T-stage PD rate were 22.2% (CI, 4.9–54.4%) and 35.7% (CI, 15.1–61.5%) of patients with T2 and T3 disease, respectively (p = 0.66). No patients with T4 disease had PD. Median time to progression was 9.0 months (range 4.6–44.3 months). FFP was similar in patients with T2 and T3 disease (p = 0.98). Overall FFP rates after 0.5-, 1- and 2-years were 95.8%, 75.4% and 68.6%, in patients with T2 disease 100%, 64.3% and 64.3% and in patients with T3 disease 92.3%, 75.2% and 62.7%, respectively. Rates of PD and FFP results were similar in patients younger and older than 66 years (p = 1 and p = 0.47, respectively). PD rates were similar in patients having SD and PD at time of TSEBT referral (p = 0.66). Patients with PD at referral had a similar FFP as patients with SD at referral (p = 0.89).
At follow-up after three months, the PD rates were 0% and 7.1% in patients with T2 and T3 disease, respectively. This PD rate was slightly increased at follow-up after one year to 14.3% and 20.0%. Twenty percent of the patients with T3 disease had PD at two-years follow-up and no patients had PD at three-years follow-up.
It is important to stress that in many cases recurrences are only in limited areas, which can easily be treated with local therapy, e.g. local steroids or local radiotherapy. Hence, the palliative effect of TSEBT often lasts considerably longer than would seem to be indicated by the FFR value.
Effect of initial therapy on TSEBT response
The difference in CR rate in patients treated with methotrexate (80.0% (CI, 55.6–94.0%)) or nitrogen mustard (72.2% (CI, 49.4–88.5%)) prior to TSEBT and patients treated with interferon (IFN-α) (55.6% (CI, 25.5–82.7%) was not statistically significant (p = 0.36 and p = 0.42, respectively). Similarly, the slightly shorter time to CR in patients previously treated with methotrexate compared to patients treated with IFN-α was not statistically significant (p = 0.34). Relapse rates were similar in patients previously treated with methotrexate (75.0% (CI, 47.1–92.4%)), nitrogen mustard (76.9% (CI, 50.3–93.0%)), IFN-α (80.0% (CI, 37.1–97.7%)) and prednisolone (87.5% (CI, 54.6–98.6%)). FFR tended to be lower in patients treated with prednisolone than in patients treated with nitrogen mustard, but the difference was not statistically significant (p = 0.32). FFR was similar in patients treated with methotrexate and IFN-α (p = 0.84) and IFN-α and prednisolone (p = 0.85). Rate of disease progression was similar in patients treated with nitrogen mustard (22.2% (CI, 8.0–44.4%)) compared to patients treated with IFN-α (33.3% (CI, 10.4–65.2%)) (p = 0.65), prednisolone (33.3% (CI, 12.5–61.2%)) (p = 0.68) and methotrexate (20.0% (CI, 6.0–44.4%)) (p = 1) prior to TSEBT. FFP tended to be higher in patients treated with nitrogen mustard compared to patients treated with IFN-α (p = 0.14) and prednisolone (p = 0.17) and similar compared to methotrexate (p = 0.78). Thus, patients treated with nitrogen mustard prior to TSEBT tended to have an improved response to TSEBT compared to patients previously treated with IFN-α and prednisolone.
Effect of maintenance therapy on duration of CR
Maintenance therapy was used to 96% of the patients and the type of treatment was chosen individually to each patient. The most commonly used maintenance therapies were topical steroids, nitrogen mustard, local radiotherapy, PUVA, IFN-α, prednisolone, methotrexate and bexarotene (data not shown). We found no statistically significant difference in FFR in patients treated with different maintenance therapies.
Side effects
Side effects related to TSEBT were observed in 88.0%. Acute side effects including erythema and ulceration were observed in 80.0%. The most common long-term, although not permanent side effects were alopecia (44.0%), dry skin (36.0%), hyperpigmentation (28.0%), ocular irritation (24.0%) and temporary loss of fingernails (16.0%). Two patients developed basal cell carcinoma after TSEBT and one squamous cell carcinoma was observed. The patient, who developed squamous cell carcinoma received PUVA prior to TSEBT, but the patients, who developed basal cell carcinoma did not receive prior ultraviolet light treatment, but one of these patients received topical nitrogen mustard. The squamous cell carcinoma was developed 45 days and the basal cell carcinomas 112 days and 1.6 years after TSEBT were finished. Thus, only one of the observed malignant skin lesions may be related to TSEBT. Plantar pain (8.0%) and chronic radiation dermatitis (4.0%) were infrequent. Systemic side effects, including bone marrow suppression, were not observed. Patients treated with low-dose TSEBT had a lower frequency of acute side effects (10.0% (CI, 1.1–38.1%)) (p < 0.001) and tended to have lower long-term side effects (30.0% (CI, 9.3–60.6%) (p = 0.05) compared to patients treated with high-dose TSEBT 76.0% (CI, 57.1–89.3%) and 72.0% (CI, 52.7–86.5%), respectively.
Discussion
In the present study high-dose TSEBT proved to be effective in the treatment of patients with CTCL with an overall response in 100% of the patients. CR was observed in 68.0% during follow-up. CR rate was similar in patients with T2 (66.7%) and T3 (78.6%) disease. No patients had T1 and only few patients had T4 disease. CR was not observed in patients with T4 disease. Median time to CR was 2.1 months and relapse following CR was observed in 82.4% with a median time of 4.0 months. One year FFP rate was 72.1% and overall 28.0% of the patients had disease progression following TSEBT.
Previous studies of TSEBT have mainly focused on treatment response in different stages of CTCL, effects of adjuvant therapies following TSEBT and doses and energies of TSEBT related to treatment outcome. Thus, impact of previous therapies on clinical response to TSEBT and the effect of clinical status before initiation of TSEBT have not been evaluated in previous studies. The majority of previous studies included patients with a lower median age than in the present study and previous results are of older date [Citation13–15]. The present study evaluated all CTCL patients in Denmark treated with TSEBT from January 2001 to November 2008 and represents updated results of TSEBT.
CR rate was similar (68.0%) to previous reports with CR rates ranging from 48.5% to 71.8% [Citation8,Citation16]. CR rate observed in the present study was slightly better in patients with T3 (78.6%) than with T2 disease (66.7%), observed OR 1.83 (CI, 0.31–10.9%). However discrepancy with an OR for T3 against T2 of 0.55 as reported by Jones et al. [Citation12] is not significant.
Prolonged disease-free survival is infrequent in patients with CTCL [Citation6]. Overall 82.4% relapsed following CR in a median time of 4.0 months in the present study. We found no difference in rate of CR or FFR between patients with T2 and T3 disease, which is in contrast to previous published studies, reporting a decreasing FFR and CR according to higher levels of T-stage [Citation14,Citation17]. This difference may be explained by the relatively low number of patients. Furthermore we found no difference in FFR in comparing patients treated with different types of maintenance therapy. To draw any further conclusions about the effect of different maintenance therapies, larger prospective studies are needed.
Patients treated with low-dose TSEBT, had a significantly lower CR rate compared to high-dose treatment. The results with low-dose therapy have previously been reported by our group [Citation8]. Furthermore low-dose therapy caused a similar relapse rate and FFR results compared to patients treated with high-dose therapy.
To our knowledge CR rate related to patient age is not reported previously. We found that patients younger than 66 years tended to have a higher CR rate, than patients older than 66 years. This is supported by the findings by Hoppe et al. [Citation15], who reported that patients with T2 disease younger than 58 years (median age) had a more favorable survival outcome than older patients. Thus, patient age could be a predictor for the extent of treatment effect and may be used in the individual assessment of treatment management.
Treatments prior to initiation of TSEBT were used in 97.1% in the present study. In patients with PD at the time of TSEBT referral CR rate and time to achieve CR were similar compared to patients with SD at the time of TSEBT referral. These results suggest that TSEBT is effective despite failure of prior therapies and that TSEBT is effective in progressive disease.
Only sparse data on PD following TSEBT is available [Citation13,Citation15,Citation18,Citation19]. Overall PD rate during follow-up was higher in the present study (28.0%) than reported by Kim et al. in patients with T2 disease [Citation15]. This may be due to inclusion of patients in all T-stages in our study as the risk of disease progression is increased with more advanced T stage [Citation20]. Similar by one year overall FFP rate was higher in the present study (72.1%) than reported from patients in late stages of CTCL (24%) [Citation13].
We investigated the impact of prior therapies on TSEBT response and found that patients treated with nitrogen mustard tended to have an improved and a prolonged response to TSEBT compared to patients previously treated with IFN-α and prednisolone. This could not be explained by differences in T-stages between these groups.
TSEBT was generally well tolerated and no systemic or persistent side effects were observed in the present study. As expected patients receiving low-dose TSEBT, had a lower frequency of side effects.
Conclusion
In conclusion, the present study confirms that TSEBT is an effective, but generally not a curative therapy in the management of CTCL. TSEBT caused a high frequency of CR, but most patients relapsed during follow-up although to a less extent of skin involvement, than before TSEBT. It remains unclear whether prior therapy to TSEBT is able to prolong the duration of the effects of TSEBT, however we did see a tendency in patients treated priory with nitrogen mustard to have a more sustained effect of TSEBT, although our data does not allow us to draw any firm conclusions. Further studies are warranted to investigate whether nitrogen mustard or any other prior therapy can prolong the effects of TSEBT.
Cutaneous T-cell lymphomas are highly radiosensitive and TSEBT offers significant palliation for patients with generalized skin disease.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lenane P, Powell FC, O'Keane C, Dervan P, O'Sullivan D, Bourke E, . Mycosis fungoides – a review of the management of 28 patients and of the recent literature. Int J Dermatol 2007;46:19–26.
- Ysebaert L, Truc G, Dalac S, Lambert D, Petrella T, Barillot I, . Ultimate results of radiation therapy for T1-T2 mycosis fungoides (including reirradiation). Int J Radiat Oncol Biol Phys 2004;58:1128–34.
- Wilson LD, Kacinski BM, Jones GW. Local superficial radiotherapy in the management of minimal stage IA cutaneous T-cell lymphoma (mycosis fungoides). Int J Radiat Oncol Biol Phys 1998;40:109–15.
- Kuzel TM, Roenigk HH, Jr., Rosen ST. Mycosis fungoides and the Sezary syndrome: A review of pathogenesis, diagnosis, and therapy. J Clin Oncol 1991;9:1298–313.
- Hoppe RT. Mycosis fungoides: Radiation therapy. Dermatol Ther 2003;16:347–54.
- Chinn DM, Chow S, Kim YH, Hoppe RT. Total skin electron beam therapy with or without adjuvant topical nitrogen mustard or nitrogen mustard alone as initial treatment of T2 and T3 mycosis fungoides. Int J Radiat Oncol Biol Phys 1999; 43:951–8.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sezary syndrome: Pathology, staging, and treatment. Curr Probl Cancer 1990;14:293–371.
- Kamstrup MR, Specht L, Skovgaard GL, Gniadecki R. A prospective, open-label study of low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2008;71:1204–7.
- Cox RS, Heck RJ, Fessenden P, Karzmark CJ, Rust DC. Development of total-skin electron therapy at two energies. Int J Radiat Oncol Biol Phys 1990;18:659–69.
- Hoppe RT, Fuks Z, Bagshaw MA. Radiation therapy in the management of cutaneous T-cell lymphomas. Cancer Treat Rep 1979;63:625–32.
- Karzmark CJ, Anderson J, Buffa A, Fessenden P, Kahn F, Svensson G, . Total skin electron therapy technique and dosimetry. AAMP Report No. 23. New York: American Institute of Physics; 1988.
- Jones GW, Tadros A, Hodson DI, Rosenthal D, Roberts J, Thorson B. Prognosis with newly diagnosed mycosis fungoides after total skin electron radiation of 30 or 35 Gy. Int J Radiat Oncol Biol Phys 1994;28:839–45.
- Funk A, Hensley F, Krempien R, Neuhof D, Van Kampen M, Treiber M, . Palliative total skin electron beam therapy (TSEBT) for advanced cutaneous T-cell lymphoma. Eur J Dermatol 2008;18:308–12.
- Hoppe RT. Total skin electron beam therapy in the management of mycosis fungoides. Front Radiat Ther Oncol 1991; 25:80–9.
- Kim YH, Chow S, Varghese A, Hoppe RT. Clinical characteristics and long-term outcome of patients with generalized patch and/or plaque (T2) mycosis fungoides. Arch Dermatol 1999;135:26–32.
- Jones GW, Hoppe RT, Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am 1995;9:1057–76.
- Wilson LD, Cooper DL, Goodrich AL, Friedman ND, Feldman AM, Braverman IM. Impact of non-CTCL dermatologic diagnoses and adjuvant therapies on cutaneous T-cell lymphoma patients treated with total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys 1994;28: 829–37.
- Jones GW, Rosenthal D, Wilson LD. Total skin electron radiation for patients with erythrodermic cutaneous T-cell lymphoma (mycosis fungoides and the Sezary syndrome). Cancer 1999;85:1985–95.
- Kim YH, Jensen RA, Watanabe GL, Varghese A, Hoppe RT. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol 1996; 132:1309–13.
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: Clinical prognostic factors and risk for disease progression. Arch Dermatol 2003;139:857–66.
