Abstract
Background. The overall completeness of the Swedish Cancer Register is high, although underreporting for certain sites must be acknowledged. The aims of the present study were twofold. Firstly to assess the completeness of reporting of pancreatic cancer to the Swedish Cancer Register, and secondly to identify and characterise long-term survivors based on information from two separate population-based register resources. Material and Methods. To assess the completeness of the Cancer Register, pancreatic cancer cases registered in the National Patient Register between 1987 and 1999 were compared to cases reported to the Cancer Register. For estimations of long-term survival, the study population was restricted to 4321 cases identified both in the Cancer Register and the Patient Register with a histopathologically confirmed diagnosis of pancreatic ductal adenocarcinoma. A complete follow-up of survival in this group was performed till December 31, 2004. Results. There was a considerable underreporting of pancreatic cancer to the Cancer Register. During the period under study, a total of 19 745 patients were identified with a diagnosis of pancreatic cancer. Of these, only 73% had been reported to the Cancer Register. The underreporting increased markedly with age at diagnosis and was more pronounced during the second period under study. Only 2.8% of patients with a histopathologically confirmed diagnosis of pancreatic ductal adenocarcinoma survived five years or longer. The likelihood of long-term survival was strongly associated with younger age and surgery. Pancreatic resection was reported in 20.4% of all patients. Median survival among those operated on was 12 months compared to 4.6 months in all patients. Conclusions. Underreporting of pancreatic cancer to the Swedish Cancer Register was pronounced and increased with older age. Less than 3% of patients with a record of pancreatic cancer both in the Cancer Register and the Patient Register survived five years or longer.
The overall completeness of the Swedish Cancer Register is high with reporting mandated by law. In a recent report based on comparisons between reported cases to the Cancer register and the records of the Patient Register and a sample survey of cancer registration in 1998, Barlow et al. estimated that a number corresponding to 3.7% of the cases reported to the Cancer Register went unrecorded [Citation1]. However, reporting was less complete for certain sites, including tumours in the nervous system, leukaemia and lymphomas and lung cancer in the elderly. In that study no assessment was made of the completeness of reporting of pancreatic cancer, a relatively rare malignancy, but a common cause of cancer death with reported mortality rates equal to or higher than incidence rates [Citation2]. Also, pancreatic cancer is one of few cancer types for which the prevalence has been reported to be lower than the incidence [Citation3].
The aetiology of pancreatic cancer remains poorly understood. Apart from increasing age and a family history of pancreatic cancer, the only established risk factors are smoking, chronic pancreatitis, and type II diabetes [Citation4,Citation5]. Pancreatic cancer has the worst prognosis of all cancers [Citation6], with a median survival of three to six months [Citation7–10]. Surgery can be considered for only 10–20% of the patients at the time of diagnosis and may increase median survival up to about 18 months [Citation11–13].
Earlier estimates of long-term survival have varied widely, which may reflect differences in patient populations under study and stage-mix [Citation12,Citation14,Citation15]. Also, methods used for reporting of survival statistics after pancreatic cancer resections have been questioned [Citation16]. Results from two recent studies have highlighted that misdiagnosis remains an issue when assessing long-term survival in pancreatic cancer patients [Citation17,Citation18]. The aim of the present study was twofold. Firstly to assess the completeness of reporting of pancreatic cancer to the Swedish Cancer Register. Secondly to identify, estimate and characterise the proportion of pancreatic cancer patients identified both in the Cancer Register and the Patient Register who had survived five years or longer after being diagnosed with a histopathologically confirmed pancreatic ductal adenocarcinoma (PDAC).
Material and methods
Data sources
Data were retrieved from the Swedish Cancer Register, the National Patient Register, and the Cause of Death Register, at the Centre of Epidemiology at the Swedish National Board of Health and Welfare. Record linkage between individuals was facilitated by the individually unique national personal registration number assigned to all Swedish residents at time of birth or first permanent residency. By means of record linkage we were able to link all incident cases of pancreatic cancers to their hospital admissions and to analyse their survival.
The Swedish Cancer Register was established in 1958 and contains information about all malignant and certain benign tumours. Both clinicians and pathologists/cytologists are required by law to report new malignancies. A cancer report has to be sent for every cancer diagnosed either clinically, by histological, cytological or other laboratory examinations, and those diagnosed at autopsy. However, information available in the Cause of Death Register is not used to add cancer cases to the Cancer Register. The overall completeness of the Cancer Register has been estimated to be 96% of all diagnosed cases, however with a relatively large variation in underreporting by diagnostic group and age [Citation1].
The National Patient Register has recorded discharges from public hospitals in Sweden since 1964 with complete national coverage from 1987 [Citation19]. Each discharge record contains up to eight discharge diagnoses coded according to the International Classification of Diseases, surgery codes, and admission and discharge dates.
The Cause of Death Register, computerised in the early 1950s, is essentially 100% complete and records dates of death as well as primary and contributing causes of all deaths [Citation20].
Classification of exposures and outcomes
All patients with pancreatic tumours were identified in the Cancer Register and the National Patient Register by the following codes: ICD10: C25 (1997–), ICD9: 157 (1987–1996).
Information retrieved from the Patient Register was used to compare registrations and to validate the diagnosis in the Cancer Register and to determine whether the patients had undergone pancreatic resective surgery (procedure codes before 1997 [Citation21]: 5510–5512, 5515, 5516; since 1997 [Citation22]: JLC10, JLC20, JLC30, JLC40).
Underlying causes of death in the Cause of Death Register were classified into four groups: Pancreatic cancer, other neoplasms (ICD9: 140–208 and 210–239, ICD10: C00–D48), diseases of the circulation system (ICD9: 390–459, ICD10: I00–I99) or any other diagnosis.
Long-term survival – study population
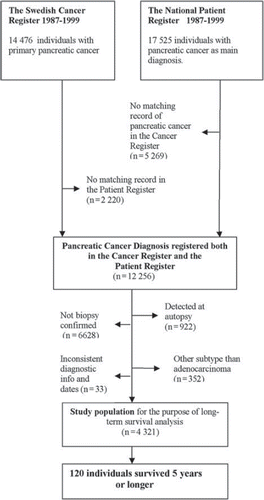
For the purpose of assessment of long-term survival, the study population was defined from cases recorded both in the Cancer Register and the Patient Register. To be included the patients had to have a record of a biopsy confirmed adenocarcinoma of the pancreas 1987–1999 in the Cancer Register and also have a recorded hospital admission with pancreatic cancer as the main diagnosis in the Patient Register. Tumours detected at autopsy were not included. Based on these inclusion criteria, the final study population encompassed 4321 patients ().
The study was approved by the Research Ethics Committee at Karolinska Institutet, Stockholm (Dnr 2006/1201-3).
Statistical analysis
To assess the reporting of pancreatic cancer to the Cancer Register, the proportion of patients reported to the Cancer Register was assessed in relation to the total number of patients identified in the Cancer Register and the Patient Register according to sex, age at diagnosis and by period of diagnosis (1987–1992, 1993–1999) and in total over the entire study period.
Moreover, Kaplan-Meier graphs were produced to compare cause-specific survival of pancreatic cancer patients identified in the Cancer Register only, the National Patient Register only, and patients identified in both databases. The Wilcoxon test was used to formally test for difference in survival across patients identified in the different data sources. Survival was calculated from the date of diagnosis in the Cancer Register or date of admission recorded in the Patient Register (whichever came first) to the date of death or December 31, 2004.
For assessment of long-term survival (defined as surviving five years or longer) cases were categorised according to age at diagnosis, period of diagnosis (1987–1992, 1993–1999), and sex. Median survival and interquartile ranges (IQR) were calculated for different sub-groups. In a separate step, patients were stratified based on duration of survival enabling us to contrast causes of deaths for short and long-term survivors. The possible influence of different characteristics on five year survival was analysed by univariate and multivariable logistic regression as odds ratios (ORs) with 95% confidence intervals (CI). The models were also adjusted for whether the patients had undergone surgery or not.
Likelihood ratio tests were used to assess statistical significance in all models. P-values less than 0.05 were considered statistically significant.
Data were analysed with Stata software version 9 [Citation23].
Results
Completeness of the Swedish Cancer Register
There was a considerable underreporting of pancreatic cancer to the Cancer Register 1987–1999. The proportion of patients reported to the Cancer Register (n = 14 476) of all identified patients (n = 19 745) was 73.3%. There were no significant differences between men or women, but the underreporting increased with age at diagnosis and was more pronounced during the second period under study ().
Table I. Patients registered in the Patient Register with a diagnosis of pancreatic cancer but not reported to the Cancer Register and the number of persons reported to the Swedish Cancer register with pancreatic cancer 1987–1999.
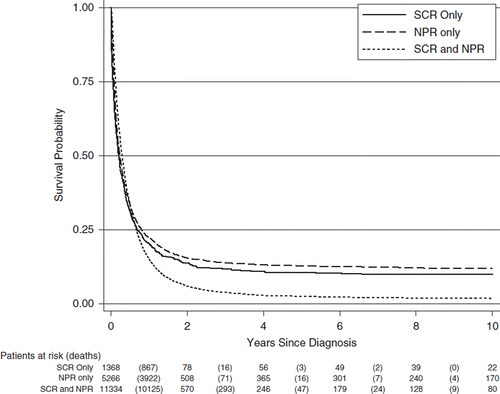
After the exclusion of cases first detected at autopsy, Kaplan-Meier survival graphs of cases reported to both the Swedish Cancer Register and the National Patient register (n = 11 334) was compared with cases only reported to the Cancer Register (n = 1368) and only reported to the Patient register (n = 5266). During the first year following diagnosis almost no difference was seen between the curves. After about 12 months a clear survival difference was observed with worse survival for patients identified in both registers. All curves were close to stable after three years with few patients dying thereafter ().
Long-term survival
Four of five (80.6%) of the 4321 pancreatic cancer patients identified in both registers had died within one year after diagnosis (). Median survival was 4.6 months (IQR 2.0–9.7) and 120 patients (2.8%) survived five years or longer. Following exclusion of non-resected long-term survivors, long-term survival was 1.9%.
Table II. Characteristics of patients with histopathologically confirmed diagnosis of pancreatic adenocarcinoma 1987–1999 by survival time (months).
Of the 120 patients who had survived at least five years, 51 (42.5%) had died at the end of the follow-up on December 31, 2004 and 37 of these from pancreatic cancer. The median survival for all deceased long-term survivors up to the end of follow-up was 7.1 years (IQR: 5.8–8.3 years). The 69 patients who were still alive at that point in time had then survived a median 9.2 years since diagnosis (IQR: 6.2–12.8 years). No major difference was observed regarding the proportion of long-term survivors during the first (1987–1992) compared to the second time period (1993–1999) ().
Cause of death
Among all cases included in the analyses of long-term survival (n = 4321), the most common recorded underlying causes of death were pancreatic cancer (94.5%), other neoplasms (2.8%), and cardiovascular diseases (1.7%). The distribution of causes of death varied between short and long-term survivors. In patients surviving five years or more, death due to causes other than pancreatic cancer was relatively more common than in short-term survivors (not shown in table).
Surgical resection
Pancreatic resections were reported in 20.4% (n = 881) of all patients identified in both registers and this proportion increased from 16.4% to 23.6% between 1987–1992 and 1993–1999. Median survival in patients operated on was 1.0 year (IQR: 0.5–2.0 years). Almost one tenth (9.4%) of the patients who had undergone resective surgery survived five years or longer, compared to 1.1% among those unresected.
Of the 120 long-term survivors, 83 (69.2%) had undergone resection. The median survival in long-term survivors who had undergone surgery and had died before the end of follow-up was 7.3 years (IQR: 5.9–8.5 years) compared to 6.2 years (IQR: 5.4–8.3 years) in those not operated on.
Among those who died within five years, the median age at surgery was 66 years (IQR: 58–72 years) compared to a median age of 63 years (IQR: 52–68 years) at surgery among long-term survivors. Surgery was more often performed in men than women (22.0% of the men and 18.8% of the women underwent resective surgery, p-value < 0.01) and among these women, 10.8% survived five years or longer compared to 8.2% of the men (p-value = 0.19).
Factors associated with long-term survival
Survival was strongly influenced by age. During the first six months after diagnosis, 71.7% of those aged 75–84 years died compared to about half of patients (48.8%) aged 64 years or younger (). This age pattern was consistent in all survival categories. Median age at diagnosis of the patients who died within five years was 68 years (IQR: 60–75 years) compared to a median age of 62 years (IQR: 52–68 years) for the long-time survivors. No overall sex differences in survival were observed.
The distribution and impact of different variables on the probability of surviving five years or longer after diagnosis are presented in . Considering all variables in a multivariable model, older age at diagnosis remained a strong and significant negative predictor of being a long-time survivor. The odds ratio for long-term survival among patients who had undergone pancreatic resection was 8.1 (95% CI: 5.4–12.2) compared to those unresected.
Table III. Background characteristics and the likelihood (expressed as odds ratios) of long- term survival among patients with histopathologically confirmed diagnosis of pancreatic adenocarcinoma (1987–1999).
Discussion
Almost one third of the patients with a record of pancreatic cancer in the Patient Register were not reported to the Cancer Register. The discrepancy was larger in the later period under study. During both periods, the underreporting increased markedly with older age and was most pronounced in the oldest age groups (> 84 years) where less than 60% of all identified cases between 1993 and 1999 were reported to the Cancer Register. A lack of correspondence between the Cancer Register and the Cause of Death Register has previously been documented in the annual Cancer Incidence in Sweden report [Citation24], where the largest differences between the records of the Cancer Register and the notifications of causes of death have been observed in elderly patients with lung cancer and pancreatic cancer. The underreporting of pancreatic cancer is also reflected in a higher mortality than incidence rate in individuals older than 70 years [Citation2,Citation3,Citation25]. The proportion of individuals without a biopsy or cytology diagnosis is probably higher among older than among younger patients. The underreporting among elderly may reflect a misunderstanding among some clinicians that only patients with cancers that have been confirmed by histology or cytology should be reported to the Cancer Register.
Our comparison of survival between the group of patients identified in both the Cancer Register and the Patient Register with survival of patients identified only in one of the registers showed almost identical pattern of survival during the first year after diagnosis. These patients were most likely correctly registered with a diagnosis of pancreatic cancer and therefore also should have been reported to the Cancer Register. After one year, survival was worse in the group of patients identified in both registers indicating that a dual reporting to the Cancer Register and the Patient Register increases the likelihood of a correct identification of patients with pancreatic cancer. After three years, few additional patients died with all three curves close to stable. In the group of surviving patients there are probably a few with other subtypes than ductal adenocarcinoma and therefore better survival, but also patients incorrectly registered with pancreatic cancer and also a few true long-term survivors.
In a recent study of the completeness of the Swedish Cancer Register, Barlow et al. [Citation1] conducted a review of medical records on a subsample of cancer cases that were recorded in the Patient Register, but not recorded in the Cancer Register. The authors found that approximately half of the identified non-reported cases should have been included in the Cancer Register. If this estimate is applied also for pancreatic cancer, it would translate into more than 2500 unreported cases during 1987–1999. Taken together, it is clear that the incidence of pancreatic cancer is underestimated in the Cancer Register. However, the underreporting to the Cancer Register is probably considerably lower for cases with a histopathologically confirmed diagnosis.
In a separate step, we focused on 4321 patients with a histopathologically confirmed diagnosis of pancreatic ductal adenocarcinoma registered both in the Cancer Register and in the Patient Register. In this group, we found a median survival of only 4.6 months which is similar to other reports based on data from population-based cancer registries [Citation7–10]. No clear differences in survival were found between men and women or over calendar-time. We identified 120 (2.8%) patients who had survived five years or longer. Most long-term survivors (69%) had undergone surgery and were younger. These estimates are in line with other recent Nordic studies reporting 1.8–3% five year survival based on registry data [Citation17,Citation18,Citation26]. However, following re-evaluation using hospital reports and pathology specimens from long-term survivors the five year survival in those studies dropped to 0.1–0.2% [Citation17,Citation18]. Our study population for estimates of long-term survival consisted of patients with a record of pancreatic cancer diagnosis both in the nationwide Cancer Register and the Patient Register. Our study design enabled a comprehensive and complete follow-up of survival and assessment of causes of death during a period of at least five years for all patients. In order to optimise the likelihood of identifying patients with a valid diagnosis of pancreatic adenocarcinoma, we performed several steps of exclusions when creating the combined data set. Only cases recorded with a histopathologically confirmed diagnosis of a pancreatic adenocarcinoma were included. Patients also had to have pancreatic cancer noted as the main discharge diagnosis in the Patient Register. With this approach, the study population that formed the basis for the survival estimates is unlikely to be fully representative of all pancreatic cancer patients diagnosed during the period under study. For example, the inclusion criteria of a histopathologically confirmed diagnosis may have led to an under- representation of older and generally ill patients. Another limitation was that no data of tumour stage, nodal involvement or metastases (TNM) were available in the Cancer Register. The records in the Patient Register included surgical codes, but no information about other treatment modalities, such as chemotherapy or radiotherapy. However, for the assessment of long-term survival of PDAC this may be less relevant. Meta-analyses of adjuvant chemotherapy have shown only a moderate increase in median survival, but not for five-year survival [Citation27], and no extra benefit from chemoradiation [Citation28]. For inoperable patients, chemotherapy and chemoradiotherapy have been reported to improve the one-year survival and quality of life [Citation29].
In the present study, one fifth (20.4%) of all patients identified in both registers had undergone surgery. This proportion is slightly higher than that observed in previous reports [Citation7,Citation8]. Almost one tenth (9.4%) of the patients with a history of surgical resection survived five years or longer. This is lower than estimates based on selected case series which have reported between 12% and 18% five year survivors after surgery [Citation11,Citation13,Citation15,Citation30,Citation31]. Earlier population-based studies have reported a median of 13 months survival after surgery compared to our finding of a median survival of 12 months [Citation7,Citation8].
Our study was restricted to information available in registries and we were not able to verify the diagnoses by re-examination of specimens. In two previous studies, re-evaluation of pathology reports and histological specimens indicated that misclassification or miscoding of the initial diagnosis was not uncommon in long-term survivors of pancreatic cancer [Citation17,Citation18]. Despite our attempts to optimise the identification of patients with a valid diagnosis (records of a diagnosis of pancreatic cancer both in the Cancer Register and in the Patient Register, biopsy confirmed diagnosis, restriction to adenocarcinomas), it is likely that cases included in the present study have been misclassified or miscoded also in the present study, especially among long-term survivors who had not undergone resective surgery. Following exclusion of non-resected long-term survivors, long-term survival in the present study was 1.9%. Also, our results from comparison of survival indicate a poorer diagnostic accuracy when data from only one register source was used.
In summary, our results show that there is a underreporting of pancreatic cancer to the Swedish Cancer Register that increases with older age. This underreporting was markedly higher than that recently reported for most other sites, including lung cancer in the elderly [Citation1]. Taken together with earlier findings from comparisons to the Cause of Death Register, it is clear that the official incidence of pancreatic cancer in Sweden is erroneously low. Also, utilising information retrieved from three separate registries, we were able to identify a small group of PDAC patients who had survived five years or longer, of which a majority had undergone surgery and were younger than the short-time survivors. Since we were unable to validate the diagnoses by re-examination, it is likely that some of these patients were misclassified and that the true proportion of long-term survivors is actually lower than the 2.8% estimate observed in the present study.
Acknowledgements
The authors wish to thank Professor Jesper Lagergren for his valuable advice and comments. The study was supported by a grant from Karolinska Institutet Research Funds. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Klint A, Engholm G, Storm HH, Tryggvadottir L, Gislum M, Hakulinen T, . Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:578–607.
- NORDCAN [Internet]. Available from: www.ancr.nu
- Batty GD, Kivimaki M, Morrison D, Huxley R, Smith GD, Clarke R, . Risk factors for pancreatic cancer mortality: Extended follow-up of the Original Whitehall Study. Cancer Epidemiol Biomarkers Prev 2009;18:673–5.
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: An update. Dig Dis 2010;28:645–56.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1766–73.
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: A population-based study (United States). Cancer Causes Control 2006;17:403–9.
- Mitry E, Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the pancreas in England and Wales up to 2001. Br J Cancer 2008;99(Suppl 1):S21–3.
- Söreide K, Aagnes B, Moller B, Westgaard A, Bray F. Epidemiology of pancreatic cancer in Norway: Trends in incidence, basis of diagnosis and survival 1965–2007. Scand J Gastroenterol 2010;45:82–92.
- Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, . Pancreatic adenocarcinoma: The actual 5-year survivors. J Gastrointest Surg 2008;12:701–6.
- Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP 2008;9:99–132.
- Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, . Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: Is cure possible? Ann Surg 2008;247:456–62.
- Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg 1987;206:366–73.
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, . Prognostic factors in resected pancreatic adenocarcinoma: Analysis of actual 5-year survivors. J Am Coll Surg 2004;198:722–31.
- Gudjonsson B. Pancreatic cancer: Survival, errors and evidence. Eur J Gastroenterol Hepatol 2009;21:1379–82.
- Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, . Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 2005;54:385–7.
- Jörgensen MT, Fenger C, Klöppel G, Lüttges J. Long-term survivors among Danish patients after resection for ductal adenocarcinoma of the pancreas. Scand J Gastroenterol 2008;43:581–3.
- The National Patient Register 2009 [Internet]. Available from: http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish
- The Cause of Death Register 2009 [Internet]. Available from: http://www.socialstyrelsen.se/register/dodsorsaksregistret/bortfallochkvalitet.
- Classification of Surgical procedures (in Swedish). Stockholm: The National Board of Health and Welfare; 1989.
- Classification of Surgical Procedures. Stockholm: The National Board of Health and Welfare; 1997.
- Stata. Stata Statistical Software. Release 9. College Station, TX, USA ed 2005.
- Cancer Incidence in Sweden 2008 [Internet]. Available from: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/17841/2009-12-1.pdf.
- Nagenthiraja K, Ewertz M, Engholm G, Storm HH. Incidence and mortality of pancreatic cancer in the Nordic countries 1971–2000. Acta Oncol 2007;46:1064–9.
- Baastrup R, Sorensen M, Hansen J, Hansen RD, Würtzen H, Winther JF. Social inequality and incidence of and survival from cancers of the oesophagus, stomach and pancreas in a population-based study in Denmark, 1994–2003. Eur J Cancer 2008;44:1962–77.
- Boeck S, Ankerst DP, Heinemann V. The role of adjuvant chemotherapy for patients with resected pancreatic cancer: Systematic review of randomized controlled trials and meta-analysis. Oncology 2007;72:314–21.
- Stocken DD, Büchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, . Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92: 1372–81.
- Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev (online). 2006;3:CD002093.
- Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, . Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–79.
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, . 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199–210; discussion 210–1.