Abstract
Background. Studies suggest an increased risk for compromised cognitive function among cancer survivors. It is unclear to what extent chemotherapy is the cause and how the dysfunction, when present, affects everyday life. The objective was to study self-reported behaviours that may depend on cognitive function, among testicular-cancer survivors who received various cycles of cisplatin-based chemotherapy by comparing them with those who did not. Material and methods. We identified 1173 eligible men diagnosed with non-seminomatous testicular cancer treated according to the national cancer-care programs SWENOTECA I–IV between 1981 and 2004. During an 18-month qualitative phase we constructed a study-specific questionnaire including questions about specific activities and behaviour in everyday life. Results. We obtained information from 960 of 1173 (82%) testicular-cancer survivors diagnosed on average 11 years previously. The prevalence of “saying similar but incorrect words“ at least once a week was 5% among those having received no chemotherapy versus 16% among those having received five or more cycles, giving a prevalence ratio (“relative risk”, RR) of 3.3 with a 95% confidence interval of 1.5 to 7.1. The corresponding figure for “saying words in the wrong order” was 3.1 (1.7–5.8), for “difficulties understanding what other people mean” 3.1 (1.3–7.7), for “saying words other than planned” 2.2 (1.1–4.5) and for “difficulties completing sentences” 2.0 (1.0–3.6). The relative risks for those with a low level of education ranged between 4.9 (1.6–14.9) and 15.3 (1.9–120.5). Conclusion. Testicular-cancer survivors in Sweden who have received five or more cycles of cisplatin-based chemotherapy experience an increased incidence of long-term compromised language; the effect is primarily seen among men with a low level of education.
The dramatic increase in the survival of patients with testicular cancer is a great success in modern oncology. Due to efficient cisplatin-based chemotherapy the cure rate has increased to more than 90% [Citation1]. The success has produced a substantial number of young male cancer survivors. We know that peripheral neurological symptoms are common among those treated with chemotherapy, even years after treatment [Citation2]. Our knowledge is, however, sparse about the potential cognitive side effects in this population.
A long-term study of Swedish twins showed that cancer survivors tend to exhibit compromised cognitive function [Citation3]. Results from breast cancer populations indicate that chemotherapy, especially in high doses [Citation4], is associated with impaired cognitive function. Available data primarily concern follow-up durations shorter than five years, and survivors have often received concomitant hormonal treatment [Citation5–8]. Testicular-cancer survivors, not exposed to hormonal manipulations, are likely to provide better opportunities for examining potential cognitive long-term effects, and their influence on everyday life, as a consequence of chemotherapy.
An expert panel agreed in a workshop that we need to learn more about possible long-term effects of chemotherapy in everyday activities related to cognitive demands, adding to the information already gained from studies based on neuropsychological testing [Citation6]. In the present study we focused on specific activities and behaviour in everyday life that may depend on cognitive function. Using this questionnaire we conducted a follow-up of Swedish men treated for non-seminomatous testicular cancer in the preceding three to 26 years. The analyses are based on a comparison between men having received various cycles of cisplatin-based chemotherapy and those not treated with chemotherapy.
Method
Study population
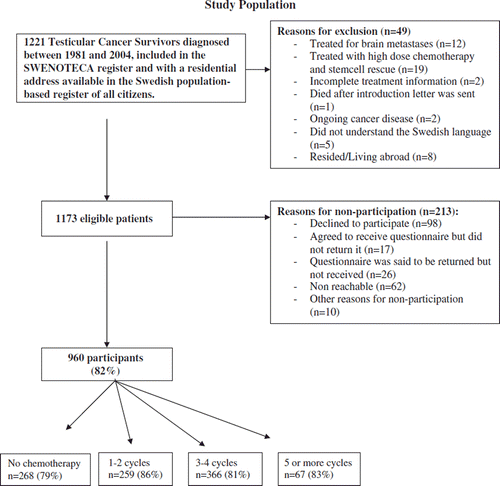
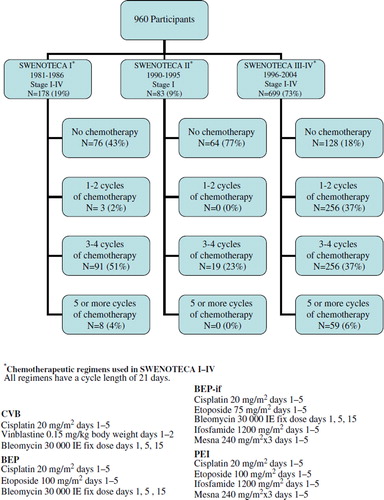
In Sweden, the handling of adult men diagnosed with non-seminomatous testicular cancer is guided by the Swedish-Norwegian cancer-care program SWENOTECA. Since 1981, with a break between 1987 and 1989, assigned clinicians have prospectively reported clinical data to the SWENOTECA database. In this database we identified 1221 living men, diagnosed between January 1981 and December 2004, who on 15 January 2007 were between 18 and 75 years old and had a residential address in the Swedish population-based register (). These men were treated according to four different SWENOTECA protocols (). The SWENOTECA database is regularly matched with the Swedish cancer register and has a close to 100% coverage of the studied population. We excluded 49 patients leaving 1173 eligible men for the analyses (). The study was approved by the research ethics committee at The Sahlgrenska Academy in Gothenburg.
Construction of the questionnaire
During an initial 18-month qualitative phase we constructed a study-specific questionnaire according to procedures developed at the Division of Clinical Cancer Epidemiology [Citation9–12]. To familiarise ourselves with problems that patients with diagnosed cognitive impairment face in everyday life, we interviewed patients (n = 13) at the Memory Clinic of Sahlgrenska University Hospital and some of their relatives (n = 7). Subsequently we interviewed 20 cancer survivors who had received cisplatin-based chemotherapy three to eight years earlier.
Using an open interview format, the first author (JS) asked for detailed examples of behaviour in various everyday activities at work, in leisure time and when performing household activities. A broad range of problems were reported, for example difficulties finding the right words, being dependent on notes to aid in remembering and feelings of lack of energy. At this stage we asked in more detail about these kinds of problems without any references to affected cognitive domains, nor did we regard it as relevant to relate experienced difficulties to treatment (chemotherapy or non-chemotherapy) in this phase. The recorded interviews were transcribed to identify distinct and concrete behaviour elements such as “word dropping” or “looking for things”. These elements were then categorised into themes such as “communicating” and “forgetfulness”. When phrasing questions for the questionnaire we deliberately tried to use the wordings given by the survivors as exactly as possible, without compromising the conceptual entity.
Response scales were developed to match each conceptual theme as carefully as possible. For questions about frequency such as “Have your words come out in the wrong order, in the last month?” we used a person-incidence scale with the verbal categories: “No”, “Yes, less than once a week”, “Yes, at least once a week”, “Yes, at least three times a week” and “Yes, at least once a day”. For questions such as “Have you had difficulties concentrating during conversations with others, in the last month?” we used a person-prevalence scale: “No”, “Yes, on some occasions”, “Yes, less than half of the time”, “Yes, more than half of the time” and “Yes, all of the time” to be more appropriate. Questions considered difficult to quantify were phrased in the following manner, “Some people have difficulties learning new phone numbers. How does that apply to you?” They were to be answered on the scale: “Do not agree at all”, “Agree to some extent”, “Agree to a large extent” and “Agree completely”. All questions were restricted to experiences during the preceding month without any reference to before or after cancer treatment.
From a total of about 800 questions retrieved from the pilot interviews we gradually reduced the number to about 300. We prioritised questions with a clear conceptual entity and those enabling the use of a person-incidence scale. To ensure that the questions and response alternatives were accurately comprehended, the first author (JS) accompanied 20 testicular-cancer survivors while they answered the questionnaire. In addition to the study-specific questionnaire, we also included questions about demographics, well-being, neurological symptoms, sexual activity, and the HADS – Hospital Anxiety and Depression Scale [Citation13]. A pilot study of 36 testicular-cancer survivors indicated what a likely participation rate would be and whether or not the men would leave certain questions unanswered. After receiving 33 of the 36 questionnaires we proceeded to the main study [Citation14,Citation15]. The final questionnaire included 295 questions sorted into 19 sections according to common themes.
Methods of data collection
Data collection proceeded during nine months from January 17, 2007 until October 18, 2007. We initially sent an introductory letter to all eligible men explaining the objectives of the study. Three to four days later telephone-calls were made to all of those whose phone numbers we found. Those who agreed to participate we mailed the questionnaire. About 10 days later they received a combined thank-you and reminder card. Fourteen days later an interviewer called those who had not returned the questionnaire, giving the informants the possibility to ask questions or decline further participation. Reminder phone calls followed for those who agreed.
Before analysing data, an expert panel [authors 1, 6, 7, 8] categorised the study-specific questions into broader cognitive domains best reflecting the main cognitive function engaged in the behaviour asked for in each question. This categorisation was first made independently by each expert followed by a group discussion until consensus was reached. We identified 59 questions mainly reflecting one specific cognitive domain; six were judged to reflect attention, 26 memory, five visual-spatial ability, seven language, two speed and 13 executive function. These 59 questions within six domains were taken to analyses in the present report.
In addition to the 59 questions about everyday life behaviour we also analysed the answers to six broader questions regarding affected well-being if having difficulties with attention, memory, visual-spatial ability, language, speed or executive function. These questions were stated in the following manner, “If you have difficulties with memory, do you experience that it has affected your well-being during the past month?”. We defined those answering “Yes, little”, “Yes, moderately” or “Yes, much” as experiences of compromised cognition, contrary to those answering “No, this does not apply to me as I have no memory problems” or simply “No”, who were defined as not having self-assessed cognitive dysfunction.
Statistics
Results are presented as relative risks, with a 95% confidence interval (95% CI), calculated as the percentage with a specific outcome (e.g. having said words in the wrong order at least once a week, the last month), among those who received one to two, three to four, and five or more cycles of chemotherapy divided by the percentage of men, with the same specific outcome, not treated with chemotherapy. Answers were dichotomised using cut-off values according to the tradition at our division of Clinical Cancer Epidemiology, reassuring that these values were reasonable according to both the nature of the question and the frequency of having an outcome in the no-chemotherapy group.
Variables potentially affecting reported experiences (potential confounders) were systematically examined, including scores on the HADS depression and anxiety scales, fatigue, retirement status, sexual lust, hearing, tumour stage and whether the patient reported retroperitoneal lymph-node dissection. The potential confounding effects was for some variables examined through restriction, i.e. when examining depression as a confounder, we calculated the relative risk for the subgroup of non-depressed participants. In the adjustment of the relative risk for variables that may reflect confounding factors, our second strategy for examining potential confounding, we utilised the GENMOD procedure with binomial distribution and log link (SAS version 9.2), i.e. logistic regression. Possible effect modifiers were examined by comparing relative risks in subgroups of, for example, different educational levels. To test for the presence of effect modification, homogeneity between strata was calculated with the Cochran-Mantel-Haenszel statistic, using the CMH option in the FREQ procedure (SAS version 9.2).
Results
Among the 1173 eligible testicular-cancer survivors, 960 (82%) answered the questionnaire. Participation rates were not statistically significantly different by number of cycles of chemotherapy (). Non-participating men and participating men had similar age at diagnosis, age at follow-up and years since diagnosis (data not shown). Demographic data are shown in . For each specific question, a few men did not respond which explains the different denominators in the tables.
Table I. Characteristics of men treated for testicular cancer during 1981 to 2004.
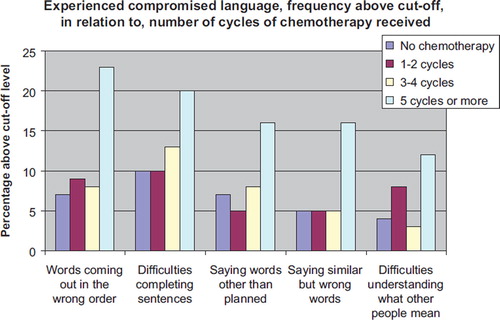
For answers to 51 of the 59 questions the confidence intervals overlapped 1.0 when we compared survivors who received five or more cycles of chemotherapy with those who received no chemotherapy. For eight questions the confidence interval did not overlap 1.0. Five of the eight were language questions, constituting five of the seven language questions in the questionnaire, with p-values ranging from 0.0002 to 0.0266 (, ). The other three were the following memory questions: “felt uncertain about what others have said or not said”, RR 2.6 (1.1–6.2, p = 0.0135), “experienced problems with memory”, RR 2.5 (1.3–4.5, p = 0.0010) and “having problems learning phone numbers by heart”, RR 1.6 (1.0–2.7, p = 0.0338).
Table II. Relative risk (prevalence ratio) and adjusted relative risks for experienced compromised language*.
When adjusting for time since diagnosis the relative risks decreased at most by 0.4, for level of education the relative risks increased or were unaffected, for anxiety the relative risks decreased at most by 0.3 (). Among other possible confounding variables examined (see Statistics) we did not find any indication of confounding (results not shown).
When examining possible effect modifiers we found the incidence of compromised language to be greatest among those with low level of education (Compulsory and Elementary school), somewhat less among those with medium level of education (High school) and least among those most highly educated (University/College). Thus, level of education modified the effects from five or more cycles of chemotherapy with p-values ranging from 0.0001 to 0.0229 for the different language questions (). Among those who received no chemotherapy we found no association between compromised language and educational level. We did not find any association between age at diagnosis and level of education (results not shown).
Table III. Relative risks (prevalence ratio) for experienced compromised language stratified for level of education*.
To separate a possible effect of different treatment strategies from a possible effect of time since diagnosis we restricted our analyses to those patients included in the SWENOTECA III-IV protocols, who had received similar treatment regiments (). When stratifying into two groups referring to time since diagnosis, and comparing those who received five or more cycles with those who received no chemotherapy, we found that the point estimate of the relative risk was greater in four of five language questions among those treated 8–11 years before follow-up than among those treated more recently. For example, the relative risk of “saying similar but wrong words” at least once a week was 4.9 (1.0–25.5) for those treated between 1996–1999 and 2.8 (0.9–9.1) for those treated between 2000–2004 (p = 0.0076).
Additionally, we analysed the answers given to the six questions regarding affected well-being if having difficulties with language, speed, memory, concentration, visual-spatial function and executive function (, ). A greater percentage of those who received five or more cycles of chemotherapy, compared to those who received no chemotherapy, reported affected well-being if having difficulties with language, memory, concentration and slower thinking speed, with relative risks ranging from 1.6 to 2.0. These outcomes were not related to either depression or anxiety scores (results not shown).
Figure 4. Affected well-being if language having language difficulties. Answers in response to the following question: “If you have difficulties with finding words or other language difficulties, do you experience that it has affected your well-being during the past month?”
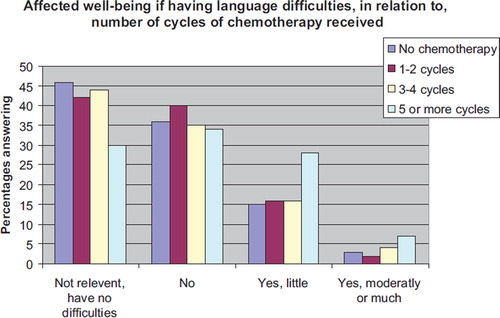
Table IV. Relative risks (prevalence ratio) for affected well-being if having difficulties with…*
Discussion
We found a greater incidence of experienced long-term compromised language among those who had received five or more cycles of cisplatin-based chemotherapy compared with those not treated with chemotherapy. The extent of this effect was related to education.
In three prior studies concerning testicular-cancer patients and cognition none included patients treated with more than four cycles of chemotherapy [Citation16–18]. On average three years after treatment, Schagen and co-workers found that cognitive problems in the domains of memory, attention, thinking and language were experienced by 22 (32%) of 70 testicular-cancer survivors who had received four cycles of chemotherapy, 18 (32%) of 57 who had received radiotherapy to abdominal lymph nodes and 15 (27%) of 55 testicular-cancer survivors who had received neither chemotherapy nor radiotherapy [Citation18]. Results for specific cognitive domains were not reported. In a European study with information from 286 of 666 testicular-cancer survivors treated with three or four cycles of chemotherapy, Fosså and colleagues found that 24% reported better and 19% worse cognition two years after treatment, compared with their pretreatment level [Citation16]. These results were based on answers given to two questions about memory and concentration from the quality of life instrument QLQ C-30 [Citation19].
The particular difficulties reported by men in our study who received five or more cycles of chemotherapy included a higher frequency of saying words other than planned, similar but wrong words and producing words in the wrong order, difficulties completing sentences and difficulties in comprehension. Downie et al. found similar types of language difficulties among 16 of 21 (78%) breast cancer patients in interviews two to six weeks after they received three to six cycles of chemotherapy [Citation20]. Evidence for compromised language function after treatment with chemotherapy was also presented in a meta-analysis performed by Stewart et al. [Citation21]. When combining results from nine individual studies on breast-cancer survivors the greatest effect sizes were observed in data from neuropsychological tests of language performance.
In our study, level of education modified the effects from five or more cycles of chemotherapy. The relationship between higher education and better cognitive function is a rather well-established fact [Citation22,Citation23]. Valenzuela et al. found that 10 of 13 studies of the association between level of education and longitudinal cognitive decline provided evidence that those with higher level of education were more resistant to age-related changes than those with a lower education [Citation22]. White et al. documented that those with the lowest level of education are most affected by cognitive impairment, those with mid-level education moderately affected and those with a college education not at all affected [Citation23]. In Alzheimer's disease patients, cognitive tests and positron emission tomography (PET) imaging studies have shown that individuals with a higher education withstand the disease better than those with a lower education [Citation24,Citation25].
We did not find any clear effects across cognitive domains for the effects of chemotherapy. As most of us are forced to use language in numerous situations in everyday life, questions about language may be a more sensitive indicator of difficulties compared to other cognitive abilities. Noteworthy, the answers given to the questions regarding affected well-being if cognitive dysfunction is at stake suggest a broader effect than indicated by the answers to the questions about behaviour (). We found a statistically significantly higher percentage amongst those who received five or more cycles, compared to those who had not received chemotherapy that reported affected well-being due to difficulties with language, memory, concentration as well as slow thinking.
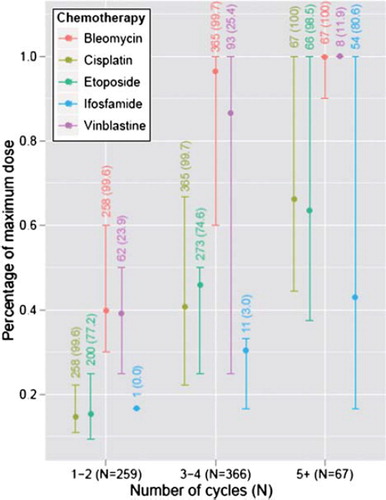
Men who received five or more cycles of chemotherapy received higher doses of cisplatin and etoposide (see ). It is possible that the cut-off between four and five cycles is a critical threshold at which the cumulative dose of cisplatin or etoposide affects language. We know platinum persists for decades in various tissues of the body after treatment [Citation26]. If it also persists in the brain it might, in common with other heavy metals, produce brain damage [Citation27]. A high percentage of those who received five or more cycles of chemotherapy were treated with ifosfamide, which very few of those who received less than five cycles received were (). “Ifosfamide encephalopathy” is an acute state occurring in up to 30% of those who receive ifosfamide [Citation28]. Confusion and delirium are common acute symptoms, suggesting that ifosfamide passes the blood brain-barrier and has immediate effects [Citation29,Citation30]. These well-documented short-term effects provide reason to suspect that ifosfamide may have long-term cognitive effects, e.g. affecting language function.
Figure 5. Minimum, maximum and mean dose, for those who received each drug respectively, standardised by dividing with the highest dose obtained. The bottom and upper part of the line stands for the minimum and maximum whereas the circle stands for the medium dose received. The numbers refer to the number and the percentage of survivors in each treatment group who have received each drug, respectively.
One can postulate that a mechanism of chemotherapy causing experienced compromised language is due to chemotherapy-induced lower serum levels of testosterone [Citation31,Citation32], or by causing more general fatigue, which in turn influences language function. Our question about “sexual desire” provided no evidence for differential effects between groups receiving varying cycles of chemotherapy. However, such a general question is neither sensitive nor specific enough compared with serum levels of testosterone [Citation33]. Moreover, long-term fatigue after chemotherapy needs to be measured more accurately than we did in our study. We cannot exclude chemotherapy cause fatigue in testicular-cancer survivors from our data.
One may believe that our results of compromised language can be accounted for by the fact that those who received five or more cycles, compared to those who received fewer cycles, were in more advanced stages of the disease. However, we found no evidence in support of this otherwise likely objection. Self-reported language difficulties among those who received five or more cycles was not related to tumour burden, retroperitoneal lymph node dissection or compromised hearing. We excluded survivors who had brain metastases.
We employed epidemiological methods as adapted to this field by the hierarchical step-model for study design and data interpretation [Citation12]. For practical reasons we restricted the study to Swedish men. To study experiences of everyday life, two different approaches can be distinguished. We can either ask direct questions about specific behaviour (e.g. “Do you say similar but incorrect words?”) or we can ask about general self-assessed function (e.g. “How is your language function?”). For the latter type of questions, researchers have documented responses may be confounded with emotional distress [Citation34,Citation35]. In accordance with what has been asked for [Citation6], and what seems more related to objective measures [Citation35–37], we chose the former approach and deliberately focused on specific activities and behaviour in everyday life. The high participation rate reduces the likelihood of selection-induced problems. Our extensive preparatory process and the use of anonymous, self-administered questionnaires mimic the technique of blinding and prevent interviewer-related bias. Our use of a study-specific questionnaire, might be considered as a weakness since it do not allow comparisons of our results with studies based on previously developed scales and questionnaires. We cannot exclude the possibility that the association between cisplatin-based chemotherapy and cognition is different in non-participants. The extent to which these results may be generalised to other populations than Swedish men may vary by genetic and cultural differences.
Detailed investigations of potential effects of chemotherapy across cognitive domains are as important in future studies as examining whether chemotherapy specifically affects language. For those men who seek help after experiencing cognitive problems following chemotherapy, communication about their experience of language function may well prove to be rewarding. Furthermore, experiences from language rehabilitation obtained from stroke and brain-injury patients [Citation38,Citation39], may be applicable also to cancer survivors treated with chemotherapy.
Acknowledgements
We thank all testicular-cancer survivors who have contributed with information, Carin Brandberg for starting up the qualitative phase of the project; Katarina Peltz, Agnetha Sundberg and Eva Quant who assisted us with the data collection, and Lawrence Lundgren for language revision. We also want to thank all Swedish uro-oncologists who since 1981 have included their patients in SWENOTECA's protocols. The study was financed by the Swedish Cancer Society (nr: CAN 2006/1350) and The West Health-care Region in Sweden.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Olofsson SE, Tandstad T, Jerkeman M, Dahl O, Stahl O, Klepp O, . Population-based study of treatment guided by tumor marker decline in patients with metastatic nonseminomatous germ cell tumor: A report from the Swedish-Norwegian Testicular Cancer Group. J Clin Oncol Epub 2011 Apr 11.
- Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA 2008;299: 672–84.
- Heflin LH, Meyerowitz BE, Hall P, Lichtenstein P, Johansson B, Pedersen NL, . Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst 2005;97:854–6.
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, . Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst 1998;90:210–8.
- Castellon S, Ganz PA. Neuropsychological studies in breast cancer: In search of chemobrain. Breast Cancer Res Treat 2009;116:125–7.
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol 2004;22:2233–9.
- Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol 2007;25:2455–63.
- Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol 2007;63:183–202.
- Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 1999;340: 1383–9.
- Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med 2004;351:1175–86.
- Steineck G, Bergmark K, Henningsohn L, al-Abany M, Dickman PW, Helgason A. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol 2002;41:244–52.
- Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol 2006;45:421–9.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70.
- Skoogh J, Steineck G, Cavallin-Stahl E, Wilderang U, Hakansson UK, Johansson B, . Feelings of loss and uneasiness or shame after removal of a testicle by orchidectomy: A population-based long-term follow-up of testicular cancer survivors. Int J Androl 2011;34:183–92.
- Skoogh J, Ylitalo N, Larsson Omerov P, Hauksdottir A, Nyberg U, Wilderang U, . ‘A no means no’–measuring depression using a single-item question versus Hospital Anxiety and Depression Scale (HADS-D). Ann Oncol 2010; 21:1905–9.
- Fossa SD, de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, . Quality of life in good prognosis patients with metastatic germ cell cancer: A prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). J Clin Oncol 2003;21:1107–18.
- Pedersen AD, Rossen P, Mehlsen MY, Pedersen CG, Zachariae R, von der Maase H. Long-term cognitive function following chemotherapy in patients with testicular cancer. J Int Neuropsychol Soc 2009;15:296–301.
- Schagen SB, Boogerd W, Muller MJ, Huinink WT, Moonen L, Meinhardt W, . Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol 2008;47:63–70.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Downie FP, Mar Fan HG, Houede-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: Evaluation with patient interview after formal assessment. Psychooncology 2006;15:921–30.
- Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol 2006;20:76–89.
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychol Med 2006;36:1065–73.
- White L, Katzman R, Losonczy K, Salive M, Wallace R, Berkman L, . Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol 1994;47:363–74.
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 2008;65: 1467–71.
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–60.
- Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, . Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000;355:1075–6.
- Troy L, McFarland K, Littman-Power S, Kelly BJ, Walpole ET, Wyld D, . Cisplatin-based therapy: A neurological and neuropsychological review. Psychooncology 2000;9:29–39.
- Ajithkumar T, Parkinson C, Shamshad F, Murray P. Ifosfamide encephalopathy. Clin Oncol (R Coll Radiol) 2007;19:108–14.
- Alici-Evcimen Y, Breitbart WS. Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology 2007;16: 956–60.
- David KA, Picus J. Evaluating risk factors for the development of ifosfamide encephalopathy. Am J Clin Oncol 2005; 28:277–80.
- Gerl A, Muhlbayer D, Hansmann G, Mraz W, Hiddemann W. The impact of chemotherapy on Leydig cell function in long term survivors of germ cell tumors. Cancer 2001; 91:1297–303.
- Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: A population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology 2006;31:565–76.
- Travison TG, Araujo AB, Beck TJ, Williams RE, Clark RV, Leder BZ, . Relation between serum testosterone, serum estradiol, sex hormone-binding globulin, and geometric measures of adult male proximal femur strength. J Clin Endocrinol Metab Epub 2008 Dec 23.
- Cull A, Hay C, Love SB, Mackie M, Smets E, Stewart M. What do cancer patients mean when they complain of concentration and memory problems? Br J Cancer 1996; 74:1674–9.
- Green HJ, Pakenham KI, Gardiner R. Cognitive deficits associated with cancer: A model of subjective and objective outcome. Psychol Health Med 2005;10:145–60.
- Ownsworth TL, McFarland K. Memory remediation in long-term acquired brain injury: Two approaches in diary training. Brain Inj 1999;13:605–26.
- Smith PC, Kendall LM. Retranslation of expectations: An approach to the construction of unambiguous anchors for rating scales. J Appl Psychol 1963;47:149–55.
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, . Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil 2000;81:1596–615.
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, . Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil 2005;86: 1681–92.