Abstract
Background and purpose. The aim of this study was to report on early effects of contemporary radiotherapy (RT) on health-related quality of life (HRQOL) and explore treatment-related contributors to the development of fatigue during RT in breast cancer (BC) patients. Material and methods. Consecutive BC patients (n = 248) referred for postoperative RT at St. Olavs University Hospital in Trondheim, Norway were enrolled from February 2007 to October 2008. Clinical- and treatment data were recorded, and HRQOL were assessed before starting (baseline) and immediately after ending RT using the “EORTC QLQ-C30” and the breast module “EORTC QLQ-BR23”. Change scores from baseline were calculated. Predictors of increased fatigue during RT were explored with multiple regression analysis adjusted for relevant confounders. Results. The global QOL- and all functional scales remained stable, except for “future perspective” which improved significantly during RT. Breast symptoms and fatigue increased significantly during RT. Groups with elevated baseline fatigue remained more stable during RT than those with lower levels at baseline. The body volume receiving 40 Gy or more (V40) was a significant predictor of increased fatigue during RT adjusted for chemotherapy, comorbidity and age (p = 0.035). Conclusion. Contemporary RT has limited early effects on HRQOL. V40 is a significant predictor of RT-related fatigue.
Multimodal treatment has become standard management of most breast cancer (BC) patients [Citation1]. Where multiple treatment modalities are involved, the contribution of each to quality of life and symptoms is difficult to quantify [Citation2]. The impact of BC treatment on health-related quality of life (HRQOL) has broadly been reported in relation to surgery techniques [Citation3] and systemic therapies [Citation4]. Improvements of radiotherapy (RT) techniques with more individual treatment plans (contemporary RT) should indicate less adverse effects and impact on HRQOL, but these assumptions have not been documented sufficiently [Citation2,Citation3,Citation5,Citation6]. Limitations of previous studies involve lack of baseline measures [Citation7,Citation8], small study samples [Citation6,Citation9] or older trials with incomparable RT-techniques to current practice [Citation5,Citation10].
Fatigue is one of the most frequent and debilitating side effects of BC treatment with adverse effects on quality of life [Citation6,Citation11–16]. The etiologies of fatigue are multi-factorial and poorly understood and the relative contributions of the various treatment modalities remain unclear [Citation14,Citation17,Citation18]. The association between the severity of fatigue and RT-variables (like dose or dose-volume) has been investigated [Citation13,Citation15], but hampered by limited samples (n = 41 and n = 52). To our knowledge no studies have tested specific hypotheses concerning RT-related contributors to fatigue developed during RT.
Prospective longitudinal study designs are generally preferred when exploring associations and predictors of post-RT fatigue [Citation11], and baseline-values of fatigue have commonly been adjusted for in regression models [Citation11,Citation14]. However, including baseline values with other explanatory variables in these study designs could lead to the bias called Lords Paradox [Citation19]. Baseline adjustment could fail to remove confounding – potentially leading to unreliable estimates of predicted effects – and, in a worst case scenario, affect that opposed to what occurs in reality [Citation20]. Small study samples and possible methodological bias in earlier studies underpin the need for further investigation of the contributors of RT-related fatigue.
The aims of this study were:
To describe HRQOL-dimensions of symptoms and functions before starting RT (baseline) and immediately after ending RT;
To report on fatigue in relation to RT for different subgroups of BC patients;
To explore possible predictors of increased fatigue during RT and illustrate a methodologically sound approach for this purpose.
Material and methods
Study design
The present work is part of a larger prospective longitudinal study where HRQOL and symptoms were registered before starting (baseline) and immediately after ending RT, and, thereafter, three, six and 12 months after baseline. This paper reports on the early subjective effects of RT.
The sample
Consecutive BC patients referred for postoperative RT at St. Olavs University Hospital in Trondheim, Norway, were enrolled in the study from February 2007 to October 2008. Exclusion criteria were metastatic disease, inability to provide informed consent, and inability to read or understand the Norwegian language. Patients prescribed chemotherapy (ChT) had completed this treatment before starting RT. Patients who developed metastatic disease were included until their disease advanced and were excluded thereafter.
RT was delivered on an outpatient basis and administered in accordance with national guidelines (Norwegian Breast Cancer Group, www.nbcg.no). Clinical target volume (CTV) after breast conserving surgery (BCS) included the mammary gland but not the chest wall muscle, whereas CTV after mastectomy included the chest wall muscle. Computer tomography (CT)-based three-dimensional (3D) dose planning was employed using Oncentra Masterplan. Local RT was delivered to the breast/chest wall (50 Gy in 2 Gy/fraction, 5 days a week) with two 6 MV photons tangential fields, frequently supplemented by low-weighted field segments to achieve optimal dose homogeneity. Patients with axillary lymph node metastases (pN +) also received 46 Gy to regional lymph nodes in the periclavicular region + /− the axillae. For this locoregional RT, the tangential fields towards the breast were half-beam matched with two wedged fields in the periclavicular/axillary region. Patients < 40 years with BCS received an additional boost dose of up to 66 Gy to the tumour bed, either with 6 MV photon fields or with a single electron field.
Data assessment – procedure
Patients were provided with oral and written study information at oncologic consultation prior RT. Baseline clinical and treatment factors were registered at the first consultation by the patients’ oncologist. Comorbidity was defined by having one or more of these chronic conditions: cardiovascular disease, pulmonary disorders, diabetes or depression. Patients with node positive disease and tumour size ≥ 5 cm were defined as a high-risk group (more advanced disease). RT-variables like treatment technique, dose/volume distributions to organs at risk and body volumes receiving high different doses were recorded. All collected data were recorded in an electronic database.
Sociodemographic variables were collected using a separate self-reported questionnaire during the first visit. High educational status was defined as having college/university education of three years or more. Level of household income was dichotomised by high income ≥ 500 000 NOK (€62 500).
Standardised self-reported HRQOL-data were collected at baseline and immediately after RT, using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 version 3.0 [Citation21] and the breast module EORTC QLQ-BR23 [Citation22]. The construction and psychometric properties of these multidimensional instruments have been well-described by others [Citation6,Citation16]. Fatigue was measured using the three-item fatigue-subscale of QLQ-C30 addressing the symptoms of tiredness, weakness and lack of energy [Citation21].
When interpreting clinically significant changes in HRQOL-variables we refer to Osoba et al. [Citation23]: difference in mean change scores about 5–10, 10–20 or > 20 points were classified as small, moderate or large, respectively.
Scoring and statistic analysis
Summary scores for the scales were calculated, and missing items were treated according to the EORTC scoring manual [Citation24]. Higher scores indicate better functioning and global health, but more symptoms. Cronbach's alpha (α) was calculated, to explore the internal consistency of the scales in our RT-sample. Mean scores (95% CI) for all EORTC scales at baseline and immediately after the end of RT were calculated. Change scores (i.e. change from baseline) with positive values indicated a worsening of symptoms but an improvement in functioning. The changes in fatigue for different subgroups were illustrated by box plots. The fatigue change score were dichotomised into “increased fatigue” and “stable/decreased fatigue”, and RT-variables were explored in these groups.
RT-related predictors of increased fatigue were explored by multiple regression analysis with stepwise entry. We hypothesised that the extent of RT (local/locoregional), axillary radiation or boost dose (yes/no), lung doses, V40 or V5 (volume of the body in cm3 that received 40 Gy/5 Gy or more) contributed to the increase in fatigue during RT. Possible confounders were co-morbidity, surgery technique, previous ChT, age and time since surgery. The final selection of explanatory variables and confounders was based on the descriptive data, clinical experience and statistical significance. The criterion for statistical significance was set at p < 0.05. Statistic analyses were carried out using SPSS Statistics Base 18.0 for Windows®.
Ethics
The main study was approved by The Regional Committees for Medical Research Ethics. All patients recruited gave their written informed consent.
Results
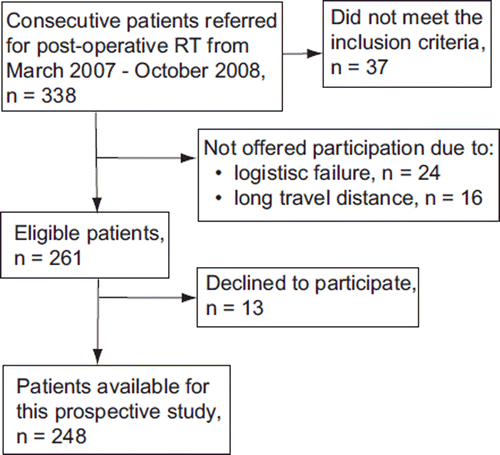
In the main study 338 patients were approached for participating, but 37 did not meet the inclusion criteria. Since participation demanded three, six and 12 month follow-ups at the hospital, 40 patients were unable to participate due to logistical problems or living at a distance (). Of 261 eligible patients, 248 confirmed their participation (95% inclusion rate). Surgery has been performed as mastectomy (27%) or BCS (73%). ChT has been administered to 41% of patients and RT was delivered locally (67%) or locoregionally (33%). Supplementary patient characteristics are presented in and .
Table Ia. Patient and treatment characteristics (n = 248).
Table Ib. Clinical and sociodemographic characteristics (n = 248).
Summary scores for all scales of the EORTC QLQ-C30 and QLQ-BR23 at baseline and after RT are shown in . The robust internal consistency of the multi-item scales was confirmed in our RT-sample by Cronbach's α values above the criterion of 0.70 ().
Table II. Mean values (95% CI) of the EORTC QLQ-C30 + QLQ-BR23 functional- and symptom scales at baseline and after RT.
The global QOL- and all functional scales remained stable, except for “future perspective” which improved significantly during RT. Thirty four percent of patients reported decreased QOL, 29% reported increased and 37% reported no change during active treatment.
Fatigue and breast symptoms increased significantly during RT, with 8.6 (CI; 5.8–11.4) and 13.5 (CI; 11.3–15.8) points, respectively. Fatigue had the highest symptom score immediately after RT. During RT fatigue increased in 53% of patients, decreased in 21% and was unchanged in 26%.
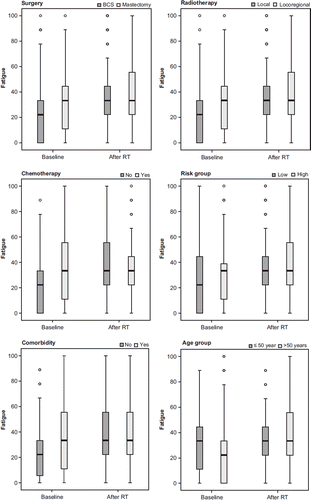
Patients, who had undergone mastectomies, received ChT or were prescribed locoregional RT or an RT boost had considerably higher levels of baseline fatigue than patients who had BCS, no prehistory of ChT or were prescribed local RT but not RT boosts, respectively. Groups with elevated baseline fatigue remained more stable during RT than those with lower levels at baseline. The latter groups displayed an increased fatigue during RT. The same pattern was seen in age-, risk- and comorbidity groups ().
Figure 2. Distribution of fatigue by surgery, chemotherapy, RT-technique, comorbidity, age and risk groups at baseline and after radiotherapy.
No significant differences in RT-variables (e.g. local vs. locoregional) between the groups with increased- and non-increased fatigue during RT were found. V40 and V5 were significant higher (p < 0.001) in patients receiving locoregional than local RT ().
Among the hypotheses concerning the contributors to RT-related fatigue, V40 was the only significant predictor to increased fatigue during RT, adjusted for ChT, comorbidity and age (). Thus, for each cubic centimeter area irradiated volume of the body receiving 40 Gy or more, fatigue tended to increase by 0.004, keeping all other variables constant. The regression model explained 16% of variability in the change in fatigue during RT.
Table III. The prediction of change in fatigue during radiotherapy, adjusted for chemotherapy, comorbidity and age.
Discussion
The purpose of this study was to report on HRQOL in BC patients during contemporary RT and to explore possible predictors of fatigue during this treatment. No significant change was seen in the mean overall QOL subscale during RT, which is congruent with other prospective studies [Citation3,Citation9,Citation16]. Patients’ outlook on the future improved substantially during RT, a finding in accordance with Lee et al. [Citation16]. Browall et al. reported a deterioration of QOL and future perspective during RT in an older sample of BC patients [Citation6], but equal levels to our patients after RT. Poorer QOL and future perspective at baseline in our sample can be attributed to more “aggressive” pre-RT treatment in younger patients. Thus, different results between Browall's and our study could be related to age.
Breast symptoms and fatigue increased significantly during RT, as shown by others [Citation3,Citation6,Citation14,Citation16]. When comparing post-RT level of fatigue with baseline, 53% reported increased fatigue during RT which is above the level reported by others [Citation15]. However, limited sample size (n = 52) in that study complicates a straightforward comparison with our results.
Patients pre-treated with ChT had a higher baseline mean level of fatigue, which remained more stable during RT than for those with no prior experience of ChT (). These findings support results published by Donovan et al.; ChT is associated with more fatigue than RT, and fatigue increases during RT only among patients not pretreated with ChT [Citation12]. This phenomenon could be interpreted as “response-shift”, defined as a change in respondents’ internal standards and values or conceptualisation of the target construct [Citation25]. The experience of unusual severe fatigue during ChT can alter patients’ perspectives on what “severe” fatigue represents or on the meaning of “fatigue as bad as it could be” [Citation11]. The same psychological processes are probably at work in patients with comorbidity and other groups with high levels of baseline fatigue (). Younger patients (≤ 50 years) also reported higher level of baseline fatigue than older patients. This is probably due to the fact that the more “aggressive” adjuvant treatment used in younger patients before RT exacerbates fatigue and other symptoms.
V40 was identified as the only significant predictor of increased fatigue during RT, adjusted for ChT, comorbidity and age (). Though V40 was significant higher in patients receiving locoregional than local RT, the large variability of V40 within the treatment groups (i.e. some patients with local treatment received large high-dose body volumes) () may explain why V40 was a predictor of RT-related fatigue while RT-technique was not. Genitz et al. found correlation between the median irradiated total tissue volume encompassed by the 50% or 90% isodose and fatigue intensity after five weeks of RT, but they believed that this was secondary to the correlation with BMI [Citation13]. However, post-RT fatigue could, in fact, be spuriously influenced by several factors. Hence, adjustment for relevant confounders should be considered when studying RT-related fatigue.
No significant effect of boost dose on increased fatigue during RT was found in our sample. Similarly, Wratten et al. stated that there were not significantly more boost-patients in a fatigued group compared to a non-fatigued group [Citation15]. However, both these findings could be biased due to few patients in the boost-groups (n = 28 and n = 20) and that the high dose areas was confined to small boost volumes.
CT-based conformal RT provides dosimetric improvements in terms of target dose homogeneity and minimisation of the volumes receiving high doses, but often at the expense of larger body volumes receiving low doses. Our findings, that high-dose volume (V40) predicts fatigue while low-dose volume (V5) does not, indicate that a transition from older techniques to contemporary conformal RT, has the potential to reduce fatigue after RT in BC patients.
The strengths of this study are its prospective design, its high inclusion rate (95%), its relative large sample size (n = 248) of a RT-population and its comprehensive evaluation and treatment of baseline measures. One limitation is that a specific fatigue scale was not employed. However, though the psychometric properties are weaker than in more extensive scales, the EORTC fatigue subscale are recommended due to its wide use, its brevity and ease of administration in larger clinical studies [Citation26]. Furthermore, the subscale has shown to be highly correlated with physical fatigue [Citation27], which probably is most prominent during RT, and demonstrated strong internal consistency (Cronbach's α = 0.90) in our RT BC sample ().
Our general findings on HRQOL are that early subjective adverse effects of contemporary RT were primarily related to breast symptoms and fatigue. Patients with prior experience of fatigue reported smaller increase in fatigue during RT than patients without such experience. We identified V40 as a significant predictor of increased fatigue during RT. Methodically sound approaches are warranted to better understand the causes of RT-induced fatigue [Citation17]. To our knowledge this is the first study exploring the contribution of RT-variables on the development of fatigue during actual treatment, adjusted for potential confounders. Our finding, that factors related to RT can explain 16% of the RT-induced fatigue, might throw light on some of the underlying mechanisms of RT-related fatigue. However, more research is needed both to understand and deal with this frequently occurring symptom.
Acknowledgements
The authors would like to thank RT technicians, physicians and nurses at the Department of Oncology at St.Olavs University Hospital for assisting with data collection, senior engineer Thorbjørn Tveit for development and operation of the electronic database and statisticians Mette Langaas and Inger Johanne Bakken for valuable statistical advice and discussion.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther 2008;83:26–36.
- Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, . A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess 2007;11:1–149, iii–iv.
- Back M, Ahern V, Delaney G, Graham P, Steigler A, Wratten C. Absence of adverse early quality of life outcomes of radiation therapy in breast conservation therapy for early breast cancer. Australas Radiol 2005;49:39–43.
- Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum 2006;33:E18–26.
- Rutqvist LE, Rose C, Cavallin-Stahl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncol 2003;42:532–45.
- Browall M, Ahlberg K, Karlsson P, Danielson E, Persson LO, Gaston-Johansson F. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. Eur J Oncol Nurs 2008;12:180–9.
- Deshields T, Tibbs T, Fan MY, Bayer L, Taylor M, Fisher E. Ending treatment: The course of emotional adjustment and quality of life among breast cancer survivors immediately following radiation therapy. Support Care Cancer 2005;13:1018–26.
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, . Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst 2004;96:376–87.
- Budischewski K, Fischbeck S, Mose S. Quality of life of breast cancer patients in the course of adjuvant radiotherapy. Support Care Cancer 2008;16:299–304.
- Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: Results of a randomized trial. Ontario Clinical Oncology Group. Cancer 2000;88:2260–6.
- Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. J Pain Symptom Manage 2009;37:341–51.
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, . Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage 2004; 28:373–80.
- Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, . Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys 2001;51:691–8.
- Noal S, Levy C, Hardouin A, Rieux C, Heutte N, Ségura C, . One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2010 (in press).
- Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O'Brien PC, . Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys 2004;59:160–7.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer 2008;16:399–405.
- Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol 2002;41:317–25.
- Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer 2008;44:1097–104.
- Lord FM. A paradox in the interpretation of group comparisons. Psychol Bull 1967;68:304–5.
- Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 2005;162:267–78.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, . The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol 1996;14:2756–68.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QOQ-C30 Scoring Manual. 3, Brussel. 2001;73.
- Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med 1999;48:1507–15.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2009;20:17–25.
- Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med 2003;17:664–72.