To the Editor,
Solitary fibrous tumour (SFT) is a rare mesenchymal tumour that arises from serosal surfaces, predominantly the pleura but also from extrapleural locations, such as the extremities, head and neck, retroperitoneum and pelvis. It often presents as a slowly growing mass and it has been associated with paraneoplastic phenomema, such as pulmonary hypertrophic osteoarthropathy (SFT of the pleura) and hypoglycaemia (SFT of the abdomen). The heterogeneity of histopathological features makes the diagnosis challenging especially when it involves differentiation from haemangiopericytomas. The two entities share many morphological and clinical similarities, so for years the terms SFT and haemangiopericytoma were used interchangeably. According to the WHO classification [Citation1], however, there are distinct histological characteristics for each subtype and they should no longer be considered part of the same spectrum. It is now acknowledged that in the majority of studies published to date tumours classified as haemangiopericytoma were in fact SFT.
There have been no prospective studies evaluating treatment modalities in SFT. Retrospective studies, however, suggest that surgical excision with clear margins should be the treatment of choice where feasible as it appears to improve survival [Citation2,Citation3]. Approximately 15–20% of cases display local recurrence or metastatic spread [Citation2–4] requiring re-excision or metastasectomy. The 10 year survival after primary surgical resection lies between 54–89% [Citation2,Citation5]. Limited published data are available on the effectiveness of systemic therapy in advanced SFT.
A retrospective search of the prospectively maintained data base at the Royal Marsden Hospital (RMH) was performed to identify patients with SFT managed with systemic treatment between 1997 and 2010. Follow-up data were censored on 31 December 2010. Ethical approval was provided by the relevant Committee at the RMH. All pathology samples were reviewed by experienced soft tissue pathologists (KT and CF).
Twenty-four patients with SFT received systemic treatment at the RMH between 1997 and 2010. These were patients with symptomatic metastatic or locally advanced disease no longer amenable to surgery and had a performance status (PS) of 0–2. The male: female ratio was 15:9 and the median age at presentation was 53 years (38–80). The primary tumour was located in the abdomen in eight cases (33%), pleura in six (25%), pelvis in four (16%), lower limb in three (12.5%), breast in one (4%), lung in one (4%) and the paraspinal area in one patient (4%). The commonest site of metastatic disease was the lung (n = 14, 58%). The vast majority of patients had surgery to the primary tumour (20/24 = 83%) and of those approximately one third (6/20) had further surgery prior to receiving systemic treatment. Tumour characteristics and surgical management are shown in . Nine patients (9/24 = 37%) received radiotherapy, six on one occasion and three in two occasions. Radiotherapy was administered either as consolidation (adjuvant) after surgery (three occasions) or as palliation for symptom control (nine occasions).
Table I. Tumour characteristics and surgical management.
Of 24 patients, 17 received anthracycline-based chemotherapy. shows the systemic therapies administered and the response to treatment. Single agent anthracycline was administered at three weekly cycles (starting dose of 75 mg/m2) in the majority of cases (11 of 14) and at two weekly cycles (starting dose of 60 mg/m2) in three cases. For all patients receiving chemotherapy the median duration of treatment was 15 weeks (3–18). At the end of treatment, over 50% of patients had progressive disease (PD), 40% had stable disease (SD) and one patient (6%) had partial response (PR). Of the seven patients receiving non-anthracycline schedules, five achieved SD (three of which were continuing on treatment at the end of the study period) and two had PD.
Table II. First line regimens for 24 patients and their response.
Severe toxicity involved: ifosfamide induced encephalopathy resulting in discontinuation of treatment at cycle 4 (one patient), doxorubicin related mucositis grade 3 resulting in hospitalisation (one patient), doxorubicin induced reduction in the cardiac ejection fraction by 20% resulting in treatment discontinuation at cycle 5 (one patient), doxorubicin induced cardiac failure after the completion of six cycles of treatment (one patient), thrombotic thrombocytopenic purpura likely related to CHR2797 resulting in discontinuation of the agent (one patient) and a transient ischaemic attack likely related to bevacizumab resulting in hospitalisation (one patient).
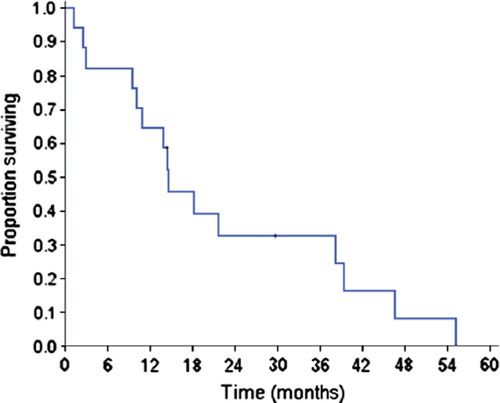
The median PFS for the chemotherapy group was 4.2 months (95% CI: 0–10.1 months). Median OS was 14.6 months (95% CI: 9.3–19.9 months) ().
Ten patients went on to have second-line treatment. Four received single agent ifosfamide (three [75%] achieved stable disease and one progressed after two cycles), and another patient was treated with KU-0059436 [a Poly (ADP-Ribose) polymerase inhibitor with antiangiogenic activity in the context of a Phase I trial] and received eight cycles of treatment over a period of 22 weeks before progression. The other patients all progressed within two months of starting second-line chemotherapy; paclitaxel (n = 1), vincristine/cyclophosphamide (n = 1), trabectedin (n = 1), imatinib (n = 1) and one with sunitinib.
Five patients received third-line treatment and all had stable disease: two had trabectedin (one with a PFS of six months), one had PM00104 (tetrahydroisoquinoline alkaloid related to trabectedin) with a PFS of five months, one had the vascular endothelial growth factor receptor inhibitor SU5416 (stable disease for 11 months) and one is currently on the VEGFR inhibitor axinitib having completed four cycles.
Overall survival from diagnosis for these 24 patients was 46 months (CI: 24.4–67.8).
In this study we report the outcomes of the largest series of patients with advanced SFT treated with palliative systemic therapy published to date. With over 50% of patients progressing on first-line chemotherapy and a median PFS of 4.2 months, this study provides evidence that conventional palliative chemotherapy is of limited value. The role of chemotherapy in SFT has been explored in small studies and case reports with conflicting results. One of the first studies suggesting that SFT might be sensitive to chemotherapy [Citation6] reported a 50% complete or partial response rate in 16 patients treated with doxorubicin alone or in combination with other agents. However, a more recent study showed only one response in a series of six patients treated with doxorubicin containing combination [Citation2]. Case reports have suggested a potential role for intraperitoneal chemotherapy in peritoneal SFT following extensive surgery [Citation7] and high dose chemotherapy with subsequent autologous peripheral blood stem cell transplantation in recurrent abdominal haemangiopericytoma [Citation8].
Our study and the others are limited by their retrospective nature and small patient numbers. However, these data show that anthracycline-chemotherapy is not effective in SFT, and that patients should not be exposed to the toxicity of such regimens. Angiogenesis inhibitors may hold promise in the management of this disease. In this study, the longest duration of clinical benefit (11 months) was observed in a patient who received a vascular endothelial factor receptor 2 kinase inhibitor in the context of a phase I study. This also highlights the potential benefit that sarcoma patients can derive from Phase I trial entry [Citation9].
The potential activity of angiogenesis inhibition has been supported by several case reports showing that treatment with interferon results in disease stabilisation [Citation10–12]. A retrospective study of 14 patients with SFT/HPC and locally recurrent or metastatic disease showed that the combination of temozolamide and bevacizumab was effective with one patient achieving partial response and 13 patients achieving stable disease [Citation13]. The PFS was 8.6 months. Other antiangiogenic agents, such as sorafenib, pazopanib and sunitinib may also be effective in advanced SFT [Citation14–16].
Conventional anthracycline based chemotherapy has minimal efficacy in advanced-metastatic SFT. International collaboration will be necessary in order to design and carry out clinical studies of novel agents in this rare sarcoma subtype.
Acknowledgements
We are grateful to the clinical nurse specialists Alison Dunlop and Cerys Propert-Lewis and the research nurses Elizabeth Barquin and Rebecca Watkins for their invaluable contribution to the management of the patients included in this study. We thank Galina Petrikova and Neera Shivakumaran for their help in the data collection and Dr Sue Ashley for assistance with the statistical analysis. We acknowledge National Health Service funding to the Royal Marsden Hospital National Institute for Health Research Biomedical Research Centre (London, UK). Dr Robin Jones is funded by the Bob and Eileen Gilman Family Sarcoma Research Program.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fletcher CD. The evolving classification of soft tissue tumours: An update based on the new WHO classification. Histopathology 2006;48:3–12.
- Spitz FR, Bouvet M, Pisters PW, Pollock RE, Feig BW. Hemangiopericytoma: A 20-year single-institution experience. Ann Surg Oncol 1998;5:350–5.
- Espat NJ, Lewis JJ, Leung D, Woodruff JM, Antonescu CR, Shia J, . Conventional hemangiopericytoma: Modern analysis of outcome. Cancer 2002;95:1746–51.
- Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer 1998;82:1915–20.
- Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, . Solitary fibrous tumors of the pleura: Clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg 2002;21:1087–93.
- Wong PP, Yagoda A. Chemotherapy of malignant hemangiopericytoma. Cancer 1978;41:1256–60.
- Peixoto Callejo I. Peritoneal solitary fibrous tumour (SFT): Long-term survival of recurrent and metastasised SFT treated with cytoreductive surgery and intraperitoneal chemotherapy. Clin Transl Oncol 2009;11:250–2.
- Kozuka T, Kiura K, Katayama H, Fujii N, Ishimaru F, Ikeda K, . Tandem high-dose chemotherapy supported by autologous peripheral blood stem cell transplantation for recurrent soft tissue sarcoma. Anticancer Res 2002;22: 2939–44.
- Jones RL, Olmos D, Thway K, Fisher C, Tunariu N, Postel-Vinay S, . Clinical benefit of early phase clinical trial participation for advanced sarcoma patients. Cancer Chemother Pharmacol 2011;68:423–9.
- Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery 2008;63:720–6; author reply 26–7.
- Lackner H, Urban C, Dornbusch HJ, Schwinger W, Kerbl R, Sovinz P. Interferon alfa-2a in recurrent metastatic hemangiopericytoma. Med Pediatr Oncol 2003;40:192–4.
- Kirn DH, Kramer A. Long-term freedom from disease progression with interferon alfa therapy in two patients with malignant hemangiopericytoma. J Natl Cancer Inst 1996;88:764–5.
- Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, . Combination therapy with temozolomide and bevacizumab in the treatment of hemangiopericytoma/ malignant solitary fibrous tumor [abstract]. J Clin Oncol 2008;26:10512.
- Ryan CW, von Mehren M, Rankin CJ, Goldblum JR, Demetri GD, Bramwell VH, . Phase II intergroup study of sorafenib in advanced soft tissue sarcomas: SWOG 0505 [abstract]. J Clin Oncol 2008;26:10532.
- Mulamalla K, Truskinovsky AM, Dudek AZ. Rare case of hemangiopericytoma responds to sunitinib. Transl Res 2008;151:129–33.
- Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schoffski P, . Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–32.