Abstract
Background. Taste alterations (TAs) are frequently reported by chemotherapy patients. However, research on this topic is very scarce. The etiologies of TAs are not fully known and prevalences may vary across tumour types and chemotherapy regimens. The aim of the present study was to longitudinally investigate TAs in patients with breast cancer or gynaecological cancers receiving chemotherapy, and to provide expected values for TAs for these patient populations. Patients and methods. One hundred and nine cancer patients (32.1% gynaecological cancer, 67.9% breast cancer) receiving chemotherapy at the Department for Internal Medicine of Kufstein County Hospital were consecutively included in the study. At each visit the Quality of Life Questionnaire-Core30 and a screening scale for TAs, consisting of two validated questions taken from the European Organisation for Research and Treatment of Cancer item bank was administered. Statistical analysis was performed using mixed-effect models. Results. The prevalence of TAs in breast cancer and gynaecological cancer patients receiving chemotherapy was high (76.1%). There were differences in the extent of TAs as well as in their time course across treatment groups. The lowest TAs were found in breast cancer and gynaecological cancer patients treated with gemcitabine. The highest TAs were found in breast cancer patients treated with epirubicin/docetaxel/capecitabine. The steepest increase of TAs was found in patients treated with epirubicin/docetaxel. Moreover, significant associations between TAs and appetite loss as well as fatigue were found. Conclusion. The results show that TAs are an issue in breast and gynaecological cancer patients receiving different chemotherapy regimens. There is a need for a more systematic investigation of TAs in chemotherapy patients in general as well as the need to address this issue in clinical practice.
Health care providers in oncology are very familiar with chemotherapy patients’ problems with taste alterations (TAs). Patients’ sense of taste can be affected quantitatively (increased or decreased perception) or qualitatively (distorted perception) [Citation1]. Thus, food often tastes different or even unpleasant and sometimes eating becomes a more and more aversive activity. On one hand, the burden from the loss of patients’ ability to enjoy meals and the loss of the associated social involvement can be severe. Taste-related problems, such as food aversions and malnutrition clearly affect daily living and emotional well-being [Citation2]. Thus, on the other hand, TAs also have to be discussed against the background of cancer cachexia, including its unfavourable effects, such as decreased response to therapy, increased mortality, enhanced morbidity of side-effects and prolonged length of hospital stay [Citation3–5].
Despite the observed frequency of this chemotherapy side-effect it has not yet been given much attention in research. The few studies allowing prevalence estimates report rates between 38% and 84% [Citation6–10], a broad range partly attributable to a diversity of concepts and measures of TAs as well as to a broad range of investigated patient groups with different diagnosis, receiving various chemotherapy regimens.
Causes for TAs in chemotherapy patients in general are manifold and to date often remain undetermined. Some authors suggest that the tumour itself contributes to the manifestation of TAs [Citation11–13]. Other factors known to influence taste sensation are poor oral hygiene, gastrointestinal reflux, infections, as well as some medications, especially antibiotics [Citation1].
TAs often start with the beginning of chemotherapy [Citation6] and persist from a few hours to weeks or even months [Citation2]. Various chemotherapeutic substances, such as folinic acid antagonists, cyclophosphamide, platines and taxanes, have been associated with TAs, predominantly with a metallic sensation [Citation1,Citation3]. Suggested mechanisms include direct effects on the epithelial cells of the taste receptor, such as receptor cell destruction or interference with cellular turnover, as well as secondary effects, such as chemotherapy induced mucositis. Some cytostatics penetrate the blood-brain barrier and might affect taste sensations in the central nervous system, e.g. by modifying afferent pathways. Further suspected causes of TAs within chemotherapy are oxidative stress and metabolic changes [Citation1].
There is little information concerning differences in the impact of different chemotherapy regimens on the severity or type of TAs. A previous study conducted within our group therefore aimed at providing expected values from TAs screening for lung, colorectal and pancreatic cancer patients receiving combination therapy with gemcitabine/platinum, etoposid/platinum, oxaliplatin/leukovorin/5-fluorouracil, gemcitabine/capecitabine, vinorelbine/platinum, or gemcitabine or irinotecan as single agents [Citation14], showing that chemotherapy type has a significant impact on the severity of TAs. The present study amends our previous work by ascertaining such expected values for chemotherapy patients with breast cancer (BC) or gynaecological cancer (GC), representing two important diagnostic groups receiving chemotherapy.
The aims of this study were: 1. Investigating the prevalence and severity of taste alterations in breast cancer and gynaecological cancer patients receiving chemotherapy; 2. Investigating the impact of clinical variables (especially chemotherapy regimen) on taste alterations; 3. Investigating time course of taste alterations across treatments; 4. Investigating the impact of taste alterations on patients’ quality of life (QOL).
Patients and methods
Sample
Patients at the Department of Internal Medicine of Kufstein County Hospital in Austria were considered as eligible for the study if they were diagnosed with BC or GC.
Inclusion criteria were: current chemotherapy (adjuvant, neoadjuvant or palliative); expected survival time of more than six months and German speaking.
Exclusion criteria were: endocrine therapy and overt cognitive impairments.
The study was approved by the Ethics Committee of Innsbruck Medical University. Sociodemographic and clinical data were gathered from hospital records.
Procedure
Data collection for this study was done within ongoing patient reported outcome (PRO) monitoring as part of clinical routine and patients were included consecutively. Assessments were done during inpatient stay or outpatient visit for chemotherapy administration. All patients were medically examined before administration of chemotherapy as a part of clinical routine. Patients provided informed consent and completed the Quality of Life Questionnaire-Core30 of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) and two questions concerning TAs on a tablet PC. A study nurse supervised data entry. For electronic PRO data capture we used software named Computer-based Health Evaluation System (CHES) [Citation15].
The measurements taken within the first week of the first cycle of chemotherapy constitute the baseline. TAs and QOL then were assessed at every in- and outpatient visit for chemotherapy administration up to 180 days after study enrolment.
Assessment instruments
EORTC QLQ-C30. All patients completed the EORTC QLQ-C30 [Citation16] which is an internationally validated and widely used QOL-instrument for cancer patients. It provides measures of functioning (Physical Functioning, Social Functioning, Role Functioning, Emotional Functioning, Cognitive Functioning), symptoms (Fatigue, Nausea/Vomiting, Pain, Dyspnoea, Sleeping Disturbances, Appetite Loss, Constipation, Diarrhoea and Financial Impact) and a scale for global QOL.
Taste Alteration Scale. As a supplement to the QLQ-C30 we added a short scale for screening for TAs, which has previously been applied [Citation14]. The scale consists of two items concerning taste and smell alterations (“Have you had problems with your sense of taste?” and “Did food and drink taste different from usual?”) which have been taken from the EORTC Quality of Life Group item bank [Citation17]. Thus, according to the QLQ-C30 style, a four point Likert scale (1–not at all to 4 –very much) is used and then scores are transformed to range from 0 to 100. The time frame the questions refer to is one week.
Based on the wording of the response categories we categorised scores of 0 points as “no taste alterations”, 16.7 and 33.3 as “mild taste alterations”, 50.0 and 66.7 as “moderate taste alterations” and 83.3 and 100.0 as “severe taste alterations”. This is a common approach in PRO studies [Citation18–20].
Statistical analyses
Descriptive statistics for the patient sample are given as percentages, means, standard deviations, and ranges. To analyse the association of the TA score with the EORTC QLQ-C30 scores we calculated Pearson correlation coefficients.
Longitudinal analysis of TAs was based on linear mixed effect models (including a random baseline term and a first order auto-regression to adjust for repeated observations from the same patient). To develop a multivariate model for the prediction of TAs with help of clinical and sociodemographic patient characteristics (see ) including time since baseline (days since first assessment) we performed a backward predictor selection. This means that we started with univariate analysis and included then all variables showing an association (p < 0.10) with TAs in a preliminary multivariate model. From this model we removed stepwise the least significant predictor until all remaining predictors showed a significant association with TAs (i.e. backward elimination).
Table I. Patient characteristics (n = 109).
Chemotherapy regimen was used as a factor with small chemotherapy regimens being collapsed to an “other regimens” category. In case of poly-chemotherapy with epirubicin, cyclophosphamide and subsequent taxanes, we added those cycles with epirubicin and cyclophosphamide to the respective group. The subsequent assessments while receiving taxanes were added to the “other” category, since it did not fit to taxane monotherapy and sample size was too small for a separate group. As reference category for parameter estimation we used the regimen with the lowest TAs score.
Results
Patient characteristics
For the study 109 were recruited (mean age 61.0 ± 12.8 years). The total number of assessments was 529 (on average 4.9 assessments per patient). About two thirds (67.9%) of patients were diagnosed with breast cancer and one third (32.1%) with gynaecological tumours (48.5% ovarian, 20.6% endometrial, 17.6% tube, 13.3% cervix). Most frequent chemotherapy regimens at baseline were epirubicin and cyclophosphamide (14.2%), epirubicin, cyclophosphamide and 5-fluorouracil (12.3%), and epirubicin and docetaxel (12.3%). No mucositis > grade 2 and no severely compromised oral hygiene were detected in our patients. Patients with infections requiring antibiotic treatment did not receive chemotherapy.
Details on sociodemographic and clinical patient characteristics are shown in .
Extent of taste alterations
To estimate severity and frequency of TAs in patients we aggregated data across all measurement time points. Mean TAs score in patients was 27.7 (SD 35.0). According to the classifications described above, no TAs were reported at 51.5% of the time points, mild TAs at 22.1% and moderate to severe TAs at 26.4% of all time points. There were 76.1% patients who reported TAs at least at one time point within the study. For further details see .
Table II. Frequency of taste alterations in patients diagnosed with breast cancer or gynaecological tumours across all time points (n = 529).
Psychometric properties of the Taste Alteration Scale
Cronbach's Alpha as a measure of reliability of the TAs scale was 0.94. No ceiling effect was found (11.3% maximum scorings).
Chemotherapy regimen and taste alterations
Patient age, time since baseline, and chemotherapy regimen were found to have a significant impact on TAs. It was decided not to include diagnostic group into the model as it is strongly reflected by chemotherapy regimen. Therefore its inclusion would have lead to strong collinearity effects.
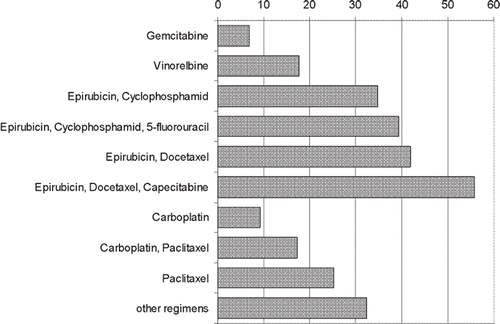
TAs increased with age (0.49 points per year) and time since baseline (18.7 points per 100 days) (). After adjusting for mean age and mean time since baseline estimated mean TAs were 6.8 for gemcitabine, 9.2 for carboplatin, 17.2 for carboplatin with paclitaxel, 17.7 for vinorelbine, 25.3 for paclitaxel, 34.7 for epirubicin with cyclophosphamide, 39.2 for epirubicin, cyclophosphamide and 5-fluorouracil (5-FU), 41.9 for epirubicin with docetaxel, and 55.8 for epirubicin, docetaxel and capecitabine ().
Table III. Mixed effect model with age, time since baseline and chemotherapy regimen as predictors of taste alterations.
Figure 1. Estimated mean taste alterations for different chemotherapy regimens (adjusted to mean age and mean time since baseline; 100 max).
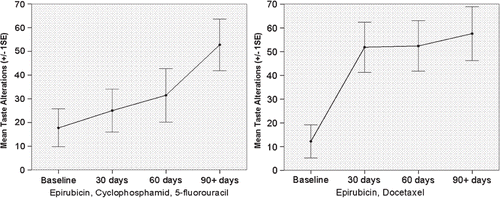
Additional analysis of the course of differences in the time course of TAs in the various chemotherapy regimens was based on the investigation of the interaction term regimen × time in the mixed effect model. These analyses revealed the strongest linear increase in patients receiving epirubicin and docetaxel (+ 18.0 points per 30 days) (observed values shown in ), followed by epirubicin, docetaxel and capecitabine (+ 17.7 points per 30 days), and epirubicin, cyclophosphamide and 5-FU (+ 9.57 points per 30 days) (observed values shown in ).
QOL domains related to taste alterations
TAs were significantly associated with impairments of certain QOL domains. The strongest correlations with TAs were found for appetite loss (r = 0.46) and fatigue (r = 0.40). Correlations between TAs and all other EORTC QLQ-C30 scales were equal or below 0.30.
Discussion
The purpose of this research was to contribute to the so far understudied issue of TAs in cancer patients receiving chemotherapy. In examining TAs in relation to various common chemotherapy regimens this study tried to provide expected values of TAs levels for patients with BC or GC undergoing chemotherapy. Clinical and socio-demographic variables, such as time since baseline and age were also taken into account. Taste influencing factors, such as antibiotics intake or severe mucositis did apply to our patients. A bacterial coating of the tongue and bacterial plaque accumulation cannot be excluded by a routine inspection of the oral cavity but due to good dental care, compromised oral hygiene is a very rare issue in Austria. Detectable problems with oral hygiene are immediately being addressed within patients’ hospital visit.
In our study sample TAs showed to be frequent (76.1% of all times points). Furthermore, 26.4% of patients reported them to be moderate or severe at least at one time point during chemotherapeutic treatment. Our results are in line with previous prevalence values reported in the literature [Citation7–9]. Lowest TAs were found in patients receiving gemcitabine (fourth line of therapy both in BC and GC), followed by patients receiving carboplatin (GC), vinorelbine (BC), or paclitaxel (BC). Our findings on gemcitabine concerning TAs are coherent with results from a previous study by Bernhardson [Citation8]. Gemcitabine generally is accepted as an agent with a favourable toxicity profile [Citation21]. The low levels of TAs in the patients receiving carboplatin and carboplatin/paclitaxel (BC and GC), are consistent with the good overall tolerability of both regimens [Citation22–24]. Concerning the investigation of poly-chemotherapies it appeared not useful to estimate the precise contribution of each single substance, as there might be interactions between the substances. We therefore focused on the overall impact of the administered regimen on the extent of TAs. All but one (carboplatin/paclitaxel) investigated poly-chemotherapies, contained epirubicin in combination with either cyclophosphamide/5-FU or docetaxel/capecitabine and were given in BC patients. These regimens were predominantly administered in neoadjuvant and adjuvant situations in BC patients. Therefore the risk of a bias through a high proportion of heavily pre-treated patients among those receiving poly-chemotherapies is low. Highest TAs were found in BC patients treated with epirubicin/docetaxel/capecitabine.
As our study provided a considerable amount of prospectively collected data, an analysis of the effect of time since baseline was performed. TAs increased considerably over the course of chemotherapy. The strongest linear increase was found in patients treated with epirubicin/docetaxel, epirubicin/docetaxel/capecitabine, and epirubicin/cyclophosphamide/5-FU. The gradient was steepest in docetaxel-containing regimens, almost reaching maximal value within the first 30 days. We can only make suppositions on underlying mechanisms. The clinical experience is that epirubicin leads to an expeditious increase of bitter and metallic taste sensations. It is detectable in saliva for days following infusion [Citation25] and may by this means have an effect on early TAs. A possible explanation for the development of TAs in docetaxel-containing regimens may be found in the higher dosage of epirubicin when given in combination with docetaxel [Citation26]. It has to be noted though, that the clinical relevance of concentrations of chemotherapeutic substances in saliva for TAs is not clarified yet.
Docetaxel itself might contribute to the maintenance of TAs by inhibiting disassembly of microtubules and thus damaging sensory nerves [Citation27]. Cyclophosphamide and 5-FU can cause TAs in a later phase of chemotherapy by inducing oxidative stress [Citation28] and mucosal toxicity [Citation29,Citation30].
Correlations of TAs with the QOL-C30 dimensions reflect some impact of TAs on daily living and well-being [Citation2]. Jensen et al. [Citation6] report that 22% of BC patients in their study who were treated with epirubicin/cyclophosphamide/5-FU or methotrexate/cyclophosphamide/5-FU rated TAs as the most disturbing oral symptom during chemotherapy. Clinical experience might lead to the assumption that the burden from TAs differs depending on treatment situation. They might become especially important in a palliative care setting where symptom control is the main focus of treatment.
The correlation of TAs with appetite loss suggests a relation with the issue of nutrition. Sanchez et al. [Citation31] found increased detection and recognition thresholds for sweet to be associated with lower daily energy and nutrients intake. In general, patients with TAs have been found to be at risk of losing weight and developing nutritional deficits [Citation32,Citation33].
However, our study does not provide information on the kind of chemosensory damage that leads to an altered taste perception, i.e. the scale does not take into account the impact of smell on the sensation of taste. The perception of flavour, which usually is meant when talking about taste non-scientifically, is strongly influenced by the sense of smell. In addition our scale does not differentiate between quantitative and qualitative TAs, nor does it provide information on the different taste qualities. In the literature there is some evidence to suggest that different chemotherapy regimens not only differ in their impact on taste thresholds but also affect different taste qualities [Citation1]. However, patients would score on the applied scale no matter which quality is affected and the main focus of the study was to assess patients’ subjective sensation of TAs. Therefore our scale serves as a viable screening tool for self-reported TAs, but detailed investigation is needed.
In general our results suggest that monotherapies are associated with lower levels of TAs than poly-chemotherapies. Since in the treatment of metastatic BC both poly-chemotherapies and monotherapies have shown to be beneficial [Citation34], QOL issues such as TAs are of particular importance in this patient group in terms of patient information and clinical decision making. Side-effects and resulting QOL impairments may affect a patients’ ability to continue treatment. This is of particular importance in a palliative care setting, where maintaining a reasonable QOL is the main treatment aim. Future research might strive for a better understanding of TAs under different chemotherapy regimens and help overcome the paucity of adequate interventions. Knowledge and management of TAs will contribute to high-quality patient information, treatment decision making and improvement of patients’ QOL.
Acknowledgements
We would like to thank Sandra Ehrenstrasser, Elisabeth Ortner and Barbara Trixl for help with data collection. Thanks also to Stefan Zugal, Jakob Pingerra and Barbara Weber for their engagement in software development. The work of Lisa Maria Wintner was partly funded by Leopold Franzens University Innsbruck. The study was supported by the Austrian National Bank “Jubilaeumsfond” the Tyrolean “Qualitätsfoerderprogramm”, and the “Verein für Tumorforschung”. The authors declare that they have no conflict of interest.
References
- Hong JH, Omur-Ozbek P, Stanek BT, Dietrich AM, Duncan SE, Lee YW, . Taste and odor abnormalities in cancer patients. J Support Oncol 2009;7:58–65.
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo Maria E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–68.
- Comeau TB, Epstein JB, Migas C. Taste and smell dysfunction in patients receiving chemotherapy: A review of current knowledge. Support Care Cancer 2001;9:575–80.
- Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 2001;23:389–98.
- Laky B, Janda M, Kondalsamy-Chennakesavan S, Cleghorn G, Obermair A. Pretreatment malnutrition and quality of life – association with prolonged length of hospital stay among patients with gynecological cancer: A cohort study. BMC Cancer 2010;10:232.
- Jensen SB, Mouridsen HT, Bergmann OJ, Reibel J, Brunner N, Nauntofte B. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:217–26.
- Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, . Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 1999;7:59–65.
- Bernhardson BM, Tishelman C, Rutqvist LE. Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer 2008;16:275–83.
- Wickham RS, Rehwaldt M, Kefer C, Shott S, Abbas K, Glynn-Tucker E, . Taste changes experienced by patients receiving chemotherapy. Oncol Nurs Forum 1999;24:697–706.
- Rehwaldt M, Wickham R, Purl S, Tariman J, Blendowski C, Shott S, . Self-care strategies to cope with taste changes after chemotherapy. Oncol Nurs Forum 2009;36:E47–56.
- Brewin TB. Can a tumour cause the same appetite perversion or taste change as a pregnancy? Lancet Oncol 1980;25:907–8.
- DeWys WD. Changes in taste sensation and feeding behaviour in cancer patients: A review. J Hum Nutr 1978;32:447–53.
- Ripamonti C, Zecca E, Brunelli C, Fulfaro F, Villa S, Balzarini A, . A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer 1998;82:1938–45.
- Zabernigg A, Gamper EM, Giesinger JM, Rumpold G, Kemmler G, Gattringer K, . Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010;15:913–20.
- Holzner B, Rumpold G. Computer-based Health Evaluation System (CHES) Version 3.0. In. 3.0 ed. Innsbruck: Evaluation Software Development; 2005.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- EORTC QLG Item Bank [Internet]. Brussels: European Organisation for Research and Treatment of Cancer. [cited 2010 Jul]. Available from: http://groups.eortc.be/qol/glossary.htm
- Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 2006;100:273–84.
- Johnsen AT, Tholstrup D, Petersen MA, Pedersen L, Groenvold M. Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol 2009;83:139–48.
- Oberguggenberger A, Hubalek M, Sztankay M, Meraner V, Beer B, Oberacher H, . Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat 2011;128:553–61.
- Lorusso D, Ferrandina G, Fruscella E, Marini L, Adamo V, Scambia G. Gemcitabine in epithelial ovarian cancer treatment: Current role and future perspectives. Int J Gynecol Cancer 2005;15:1002–13.
- Guarneri V, Piacentini F, Barbieri E, Conte PF. Achievements and unmet needs in the management of advanced ovarian cancer. Gynecol Oncol 2010;117:152–8.
- Covens A, Carey MS, Bryson P, Verma s, Fung Kee Fung M, Johnston M. Systematic review of first-line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecol Oncol 2002;85:71–80.
- Ozols RF, Bundy BH, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, . Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194–200.
- Dodde WIW, Maring JG, Hendriks G, Wachters FM, Groen HJM, de Vries EGE, . Determination of epirubicin and its metabolite epirubicinol in saliva and plasma by HPLC. Ther Drug Monit 2003;25:433–40.
- Gluck S. Adjuvant chemotherapy for early breast cancer: Optimal use of epirubicin. Oncologist 2005;10:780–91.
- Pellegrini F, Budman DR. Review: Tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest 2005;23:264–73.
- Look MP, Musch E. Lipid peroxides in the polychemotherapy of cancer patients. Chemotherapy 1994;40:8–15.
- Kennedy BJ. 5-fluorouracil toxicity: Old or new? Cancer 1999;86:1099–100.
- De Placido S, Lopez M, Carlomagno C, Paoletti G, Palazzo S, Manzione L, . Modulation of 5-fluorouracil as adjuvant systemic chemotherapy in colorectal cancer: The IGCS-COL multicentre, randomised, phase III study. Br J Cancer 2005;93:896–904.
- Sanchez-Lara K, Sosa-Sanchez R, Green-Renner D, Rodriguez C, Laviano A, Motola-Kuba D, . Influence of taste disorders on dietary behaviors in cancer patients under chemotherapy. Nutr J 2010;9:15.
- Mattes RD, Arnold C, Boraas M. Learned food aversions among cancer chemotherapy patients. Incidence, nature, and clinical implications. Cancer 1987;60:2576–80.
- Lees J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur J Cancer Care (Engl) 1999;8:133–6.
- Beslija S, Bonneterre J, Burstein HJ, Cocquyt V, Gnant M, Heinemann V, . Third consensus on medical treatment of metastatic breast cancer. Ann Oncol 2009;20:1771–85.