Abstract
Background. Breast cancer during pregnancy (BCP) is relatively rare and is associated with controversies about its biology and prognosis. Hence, we designed a case-control study to examine tumor features and outcome in a series of BCP patients diagnosed and treated in a single institution. Material and methods. We identified 65 patients diagnosed with BCP and for each; we selected two non-pregnant breast cancer patients, who were matched for age, year of surgery, stage, and neoadjuvant chemotherapy. We then compared the differences in pathology, immunohistochemical features (ER, PR, HER2 and ki-67), disease-free (DFS) and overall survival (OS). Results. We did not find any significant differences in tumor characteristics between the two groups. However, at a median follow-up of four years, BCP patients had an inferior DFS (HR 2.3; 95% CI 1.3–4.2), after adjustment for possible confounding covariates. No difference in OS was observed. However, upon restricting the analysis to patients who did not receive neoadjuvant chemotherapy, patients with BCP had inferior OS as well (HR 2.6; 95% CI 1.0–6.5). No association between induction of abortion and prognosis was observed. Conclusions. While we did not observe any differences in tumor features, BCP patients have poorer prognosis compared to age and stage-matched control. Further studies should try to elucidate reasons for such poor outcome.
Breast cancer (BC) is the most common invasive malignancy occurring during pregnancy [Citation1]. It is estimated that around 10 000 patients are diagnosed every year with BC during pregnancy (BCP) worldwide [Citation2]. This disease represents a real challenge, because applying standard therapies is not possible at all times and could potentially endanger the course of pregnancy and fetal outcome [Citation3]. Induction of abortion is not always acceptable for several social and ethical reasons as well. In addition, it is unknown whether it improves prognosis [Citation4].
During the past decade, several groups and a recent consensus meeting have suggested strategies to manage patients diagnosed with BCP [Citation5–8]. Yet, considerable controversy exists regarding the tumor characteristics and prognosis of these patients. This remains highly clinically relevant, as it could potentially refine our treatment approaches to these highly challenging cases. To date, several case-control studies have been conducted to address the prognosis of BCP patients [Citation9–14]. The majority of these studies suffer major limitations, including the lack of proper matching according to important prognostic parameters, limited information on tumor characteristics, small sample sizes and long recruitment periods. In addition, the definition of BCP was not consistently defined across the different studies, and patients diagnosed with BC six to 12 months following delivery were often included. This has resulted in conflicting results about whether pregnancy influences BC outcome.
In contrast to the foregoing, the impact of pregnancy on BC biology was seldom investigated. A very few case-control studies have looked into the differences in estrogen (ER) and progesterone receptors (PR) between patients with BCP and matched controls, but they have also produced inconsistent findings [Citation9–11].
Hence we conducted a case-control study to compare the differences in biological features at the pathological as well as the genomic levels between patients diagnosed with BCP and matched BC controls. Here we report the differences in the pathology, immunohistochemical features and prognosis between the two groups.
Material and methods
Patient selection
We retrospectively reviewed the records of women ≤ 50 years of age who were diagnosed and treated at the European Institute of Oncology (IEO) in Milan in the period from January 1996 until October 2010. Patients with BCP were defined as those who were clinically and/or histologically diagnosed during pregnancy. Patients who were clinically diagnosed at the IEO with BC during pregnancy but whose tissue biopsy was delayed until after delivery were included. However, patients who were clinically diagnosed anytime following delivery were not considered eligible for this study. For each patient, we identified two BC controls who were not diagnosed during pregnancy or lactation and were matched according to age (± 2 years), year of surgery (± 2 years), pathological tumor size (pT), and pathological nodal status (pN). As matching according to pT and pN would not be accurate in patients treated with neoadjuvant chemotherapy, we considered neoadjuvant chemotherapy as a matching criterion as well in our study. All patients agreed to have their clinical data and tumor specimens used for the purpose of this study as per institutional policy.
Treatment
The ultimate decisions regarding treatment strategy, pregnancy management and the use of systemic therapies were based on clinical staging, pathological parameters, physicians’ and patients’ choices. All patients received definitive loco-regional treatment with breast-conserving surgery or mastectomy with or without radiotherapy. In pregnant patients who required adjuvant radiotherapy, this was postponed until delivery. Sentinel node biopsy was routinely performed when appropriate, even in patients diagnosed during pregnancy, as previously reported [Citation15]. In pregnant patients, chemotherapy was always administered after the first trimester. A small number of the pregnant patients were previously enrolled in a clinical study conducted by our group investigating single agent weekly epirubicin during pregnancy [Citation7].
Pathological assessment
All patients were pathologically diagnosed at the IEO. For the sake of this study, all pathological specimens of eligible patients were reviewed. Pathological assessment included evaluation of the histological type, histological grade and peritumoral vascular invasion (PVI). Tumor grade was evaluated according to Elston and Ellis [Citation16] and PVI was assessed as previously reported [Citation17]. Estrogen receptor (ER), progesterone receptor (PR), ki-67 labeling index and HER2 expression were evaluated by immunohistochemistry (IHC), as previously described [Citation18]. Briefly, the tumor sections were incubated with the specific primary mouse monoclonal antibodies to ER (clone 1D5, 1:100 dilution; Dako, Glostrup, Denmark) and PR (clone 1A6, 1:800 dilution; Dako). Ki-67 was assessed using the MIB1 monoclonal antibody (1:200 dilution; Dako), while HER2 was evaluated using the polyclonal antibody by Dako (1:1600 dilution). The percentage of neoplastic cells showing definite nuclear immunoreactivity out of 2000 cells was recorded for ER, PR and Ki-67. For HER2 assessment, tumors were scored according to the intensity and completeness of cell membrane staining, in a 4-tier scale (0: no immunoreactivity; 1 + : weak and incomplete membrane staining; 2 + : weak/moderate and complete membrane staining; and 3 + : strong and complete membrane staining); furthermore, the percentage of immunoreactive neoplastic cells was recorded. Tumors scored 3 + were considered as overexpressing HER2. Florescence in situ hybridization (FISH; PATHvision HER2 DNA probe kit, Abbott, Des Plains, IL, USA) was undertaken for tumors with a HER2 score of + 2 by IHC.
Tumors were divided into four BC subtypes: luminal-A, luminal-B, HER2-positive and triple-negative (TN) using immunohistochemical surrogate markers as previously defined [Citation19]: luminal-A tumors were defined as ER (and/or PR)-positive, HER2-negative with low Ki-67 labeling index (< 14%); luminal-B tumors were ER and/or PR-positive and had high Ki-67 labeling index (> 14%) or HER2 overexpression/amplification; HER2-positive subtype included non-luminal HER2-positive tumors; TN subtype included those with negative expression of ER, PR and HER2.
Statistical analysis
The association between pregnancy and the clinico-pathological characteristics was analyzed using the χ2 test or the Fisher exact test, as appropriate. Survival endpoints were disease-free survival (DFS), BC-related disease-free survival (BRDFS) and overall survival (OS). DFS was calculated from the date of surgery to any loco-regional or distant recurrence, contralateral BC, other primary tumor or death from any cause, whichever occurred first. BRDFS was calculated from the date of surgery to any loco-regional or distant recurrence or death from BC, whichever occurred first. OS was calculated from the date of surgery to death from any cause. In the absence of any of the above-mentioned events, survival was censored at the last follow-up visit or phone call with the patient. Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test. The independent prognostic impact of pregnancy on survival was evaluated using multivariate Cox proportional hazard regression models and expressed as hazard ratio (HR) with 95% confidence intervals (CI). Each model was adjusted for the variables that were statistically significant in the univariate analysis. Analyses were performed with the SAS software version 9.2 (SAS Institute, Cary, NC, USA) and R software Version 2.12.2 (available at www.r-project.org). All tests were two-sided.
Results
Patients’ characteristics
A total of 65 patients with BCP were eligible, for whom we identified 130 patients to serve as controls. All BCP patients were pathologically diagnosed during pregnancy except nine patients who were clinically diagnosed at our institution during the pregnancy course, but for whom pathological diagnosis was made following pregnancy termination. summarizes the patients’ characteristics. The median age at diagnosis was 36 years (range: 28 to 47) and around 40% of patients had pT1 and node-negative disease. Only 11% of patients received neoadjuvant chemotherapy in both groups. Forty-four (68%) and 81 (62%) patients were treated with systemic chemotherapy in the pregnant and control groups, respectively. The majority were treated with an anthracycline-based regimen. Of note, single agent weekly epirubicin was administered in 13 BCP patients during pregnancy. The latter patients were shifted to more standard regimens following delivery. As of June 2005, all patients with HER2-positive BC were treated with adjuvant trastuzumab. In the pregnant group, trastuzumab was given only following delivery.
Table I. Summary of patients ’ characteristics showing matching between the two groups according to age at diagnosis, year of surgery, pT, pN and neoadjuvant chemotherapy. There were no differences observed in the type of surgery, adjuvant systemic therapy or radiotherapy.
Pathology assessment
Fifty-eight patients (89%) with BCP were diagnosed with invasive ductal carcinoma NOS, while no lobular carcinomas were observed. The majority (55%) had poorly differentiated tumors, and the most common BC subtype within the BCP group was luminal-B HER2-negative (49.2%) (). In comparison to the control group, we did not observe any differences in the pathological features, or IHC analyses ().
Figure 1. The distribution of breast cancer (BC) subtypes in patients diagnosed with breast cancer during pregnancy (BCP) and matched BC controls. No differences were observed (p50.676).
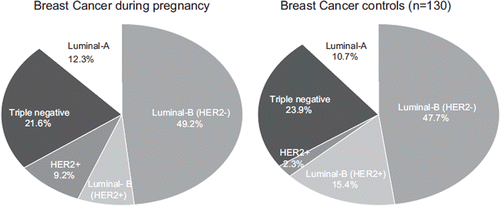
Table II. Pathological features of patients with breast cancer during pregnancy (BCP) and matched breast cancer (BC) controls. No significant differences between the two groups were observed.
Difference in survival between BCP patients and matched controls
At a median follow-up of four years (range 2–153 months), 19 (29%) and 11 (17%) patients with BCP relapsed and died respectively, compared to 27 (20%) and 15 (11%) patients in the control group. The differences in events between the two groups are summarized in . The main difference observed was the higher rate of distant metastases (23.1% vs. 13.8%) found in the BCP group.
Table III. Differences in events between patients with breast cancer during pregnancy (BCP) and matched breast cancer (BC) controls. More distant relapses were observed in the BCP group.
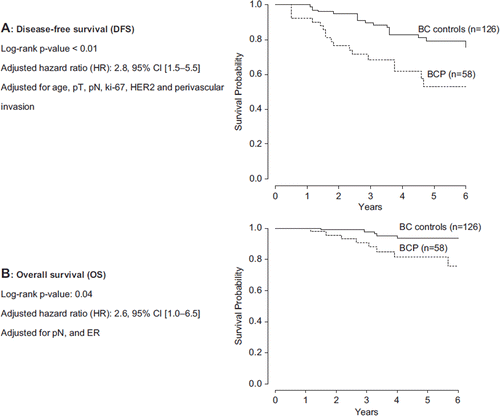
Using the log-rank test, BCP patients had a significantly inferior DFS (5-year, 52.1% vs. 74.3%; p = 0.01) and BRDFS (5-year, 56.6% vs. 74.3%; p = 0.04), even after adjustment for age, pT, pN, neoadjuvant therapy, Ki-67, HER2, and PVI (). Hazard ratios for DFS and BRDFS were 2.3 (95% CI 1.3–4.2) and 2.0 (95% CI 1.1–3.7), respectively. As for OS, BCP patients had a tendency towards inferior survival, but the difference was not statistically significant (5-year OS, 79.6% vs. 88.4%; p = 0.17) ().
Figure 2. Differences in DFS and OS between patients with breast cancer during pregnancy (BCP) and matched breast cancer (BC) controls. A. disease-free survival, B. overall survival.
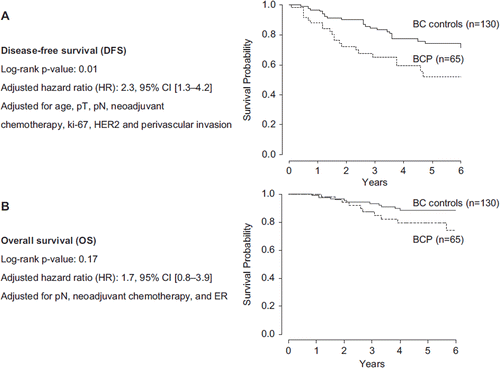
Given that survival endpoints were calculated from the date of surgery, and matching according to pT and pN was not possible in patients receiving neoadjuvant chemotherapy, we performed a sensitivity analysis by excluding these patients (11%) in an attempt to have a more homogenous patient population. We obtained the same results, but with a borderline significant difference in OS as well (HR: 2.6; 95% CI 1.0–6.5; p = 0.04) ().
Association of pregnancy termination with BC outcome
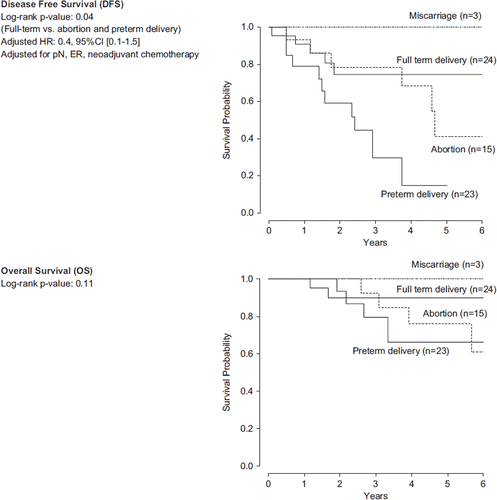
We examined whether abortion or preterm delivery was associated with BC outcome. Of 65 patients with BCP, 15 (23%) had a full-term delivery at a gestational age ≥ 37 weeks, 32 (49%) had a preterm delivery, 15 (23%) had an induced abortion and three (5%) had miscarriage. Of those who delivered before week 37 of gestation, seven patients had an early preterm delivery at gestational age ≤ 33 weeks. After exclusion of patients who experienced miscarriage (n = 3), patients who had a full-term delivery showed a better DFS compared to those who delivered preterm or were subjected to induced abortion (p = 0.04). However, after adjusting for differences in pN, ER and neoadjuvant chemotherapy, the difference was no longer significant (HR: 0.4; 95% CI 0.1–1.5) (). No difference in OS was observed (p = 0.11) ().
Impact of treatment delays
Within the BCP group, 23 patients (35%) started adjuvant systemic therapy within two months after BC diagnosis, and 42 (65%) patients did so more than two months after. Adjusting for pN and ER, we did not observe any detrimental effect of treatment delay on DFS (HR 0.6; 95% CI 0.2–1.8) or OS (HR 1.6; 95% CI 0.4–7.4). (Supplement 1). Supplementary Figure 1: available in the online version of the journal. Please find this material with the direct link to the article: http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.636069).
Discussion
The results of our study show that the pathological features of patients with BCP appear to be similar to matched BC controls. However, and despite applying very stringent matching criteria, patients with BCP had poorer prognosis than the control group.
Early studies suggested that the poor prognosis associated with BCP is secondary to diagnostic delay, which results in a high incidence of advanced disease at presentation [Citation13,Citation20], and has a poor impact on prognosis. We opted to select controls matched for tumor size and nodal status to ensure that the control group had exactly the same stage; nevertheless, we observed a worse outcome as well.
A multicentric Brazilian study involving 87 patients with pregnancy-associated BC and 252 age-matched controls has been recently published [Citation12]. In this study, there was no matching according to stage, and around 40% of patients had no available information on ER. In addition, patients diagnosed with BC within one year following pregnancy were eligible. The study showed that patients with pregnancy-associated BC had a significantly inferior median survival when compared to controls (30.1 vs. 53.1 months; p = 0.005). This was observed even after adjustment for histological subtype, grade, ER status and tumor size (p = 0.011). Another large study by the California Cancer Registry including 779 patients and 4177 age-matched controls showed the same results [Citation11]. Patients with BC diagnosed one year following pregnancy were also eligible, and more than 30% of patients had missing information on hormone-receptor-status. After adjustment for potential confounding covariates, patients with pregnancy-associated BC had a poorer survival (p = 0.04). In contrast, other studies did not show a negative impact of pregnancy on BC outcome [Citation9,Citation14]. A study from MD Anderson included around 100 BC patients diagnosed during pregnancy and lactation and 550 age-matched controls showed a higher risk of distant metastases in the former group (45% vs. 38%) but the difference was not statistically significant [Citation9]. Of note, this study included patients treated over a period of 33 years (1973–2006). In addition, there was no matching according to stage and around 25% of patients had missing information on hormone receptor status. Our study has the advantage of being mono-centric, with stringent matching criteria, homogenous patient population and complete pathological and IHC information. Moreover, it describes a recently treated series of patients. These factors were seldom combined in any of the previously reported studies.
The prognostic impact of induced abortion remains unclear. Some physicians advocate abortion in order to apply standard treatment strategies without having to worry about their potential pregnancy-related toxicities. Others might suppose that the continuation of pregnancy could aggravate the aggressiveness of the tumor because of hormonal stimulation. However, none of these concerns are evidence-based. In our study, we did not observe improved outcome with induction of abortion or delivery anticipation. On the contrary, they appeared to correlate with worse outcome. However, after adjusting for possible confounding covariates, the differences were no longer significant. This is probably related to the fact that such approaches were mainly performed in patients with advanced stage and/or aggressive tumors. Similar results were recently presented by Loibl et al. on reporting the results of a BCP European registry project [Citation21]. In this study, patients who underwent abortion had an inferior DFS (p = 0.03). However, the analysis was not adjusted for potential confounding factors. While we acknowledge that the number of patients is small to draw solid conclusion, our results suggest that abortion does not appear to improve the prognosis of BCP patients and hence it should not be advocated for this purpose.
In an attempt to explain the difference in survival observed in our study, we tried to interrogate other factors that could have had an effect on the prognosis of BCP patients. We have observed that all patients in the control group received adjuvant therapy within two months from the date of diagnosis compared to only 35% of patients in the BCP group. This could indeed have an impact on the poorer prognosis of the BCP group. However, we did not observe in the BCP group any survival difference between patients treated within two months of BC diagnosis and those treated later than two months. This is explained by the enrichment of the delayed-treatment group by patients with early stage ER-positive disease who were offered hormonal therapy alone, which was deferred until delivery given the treatment's high teratogenicity when taken during pregnancy [Citation4].
In the BCP group, 13 patients received single agent weekly epirubicin as (neo)adjuvant therapy at a dose of 35 mg/m2 as previously reported by our group [Citation7]. However, this regimen is not standard outside of pregnancy, and it could be claimed that it is inferior to more standard ones [Citation22]. Of note, weekly epirubicin was given for a median of 12 weeks during pregnancy, and patients were shifted to more standard regimens following delivery. To rule out a possible detrimental effect of using weekly epirubicin, we compared the outcome of patients treated with this regimen during pregnancy and those treated with others, yet we did not observe any differences in DFS or OS (data not shown).
We hypothesized that pregnancy could alter the biology of BC and thus started by examining the differences in pathological features and the distribution of BC subtypes by IHC. To the best of our knowledge, none of the previously published case-control studies have described the distribution of BC subtypes or even the differences in HER2 and ki-67 expression. However, we did not observe any significant differences between the two groups.
It is possible that pregnancy alters BC biology by exerting an effect on the mammary stem cells. During pregnancy, there are high levels of growth hormone (GH), and GH/insulin growth factor-1 axis has been shown to serve as a master regulator coordinating cells with stem cell features in various organs, including the breast [Citation23]. Interestingly, it has been shown that pregnancy induces a transient 11-fold increase in mammary stem cell numbers and that these cells overexpress GH receptor [Citation23,Citation24]. Another view is that pregnancy could potentially exert an effect on the biology of BC via its effect on the micro-environment, particularly the breast stroma. It is not hard to imagine that the breast stroma in young pregnant women is highly responsive to growth factor stimulation given the requirements of the breast to accommodate pregnancy and hence this microenvironment could also be advantageous for aggressive tumor growth [Citation25].
This study suffers some limitations that should be taken into account. The size of the study does not allow drawing solid conclusions regarding the prognostic impact of abortion. However, it runs in line with the preliminary results of the European Registry Project mentioned earlier. Also, we observed a difference in OS which was only significant after exclusion of patients receiving neoadjuvant therapy. This might raise some questions on the robustness of this result given that response to neoadjuvant therapy in patients with BCP has been recently suggested to be equivalent to matched BC patients [Citation26]. However, it is important to note that neoadjuvant therapy is not solely offered in BCP due to advanced clinical stage as in the case of BC controls. In certain circumstances related to gestational age of pregnancy, delay of surgery is preferred in some cases. Hence we opted to perform a sensitivity analysis to ensure having more homogenous and matched population in both groups. Nevertheless, our study shows that BCP is independently associated with a higher risk of relapse despite the perfect matching according to the different prognostic factors and apparent similarities in pathological features.
In summary, this study refines our understanding to the biological features and prognosis of patients diagnosed with BCP. We believe that pregnancy exerts an effect on the biology of these tumors that is not captured using conventional pathological techniques. To confirm this assumption, we are planning to interrogate several aberrations at the genomic and epigenetic levels to elucidate the biological and prognostic features of this relatively rare, poorly investigated, and yet very challenging disease.
http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.636069
Download PDF (212.3 KB)Acknowledgments
The authors would like to thank Carolyn Straehle for her editorial assistance during the preparation of the manuscript and Elena Ciriello for data collection. There is no conflict of interest to be declared. This work was supported by the 2010 Avon Run in Milan, Italy. Hatem A. Azim Jr. is supported by a translational research grant from the European Society for Medical Oncology (ESMO).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am J Obstet Gynecol 2003;189:1128–35.
- Pentheroudakis G, Pavlidis N. Cancer and pregnancy: Poena magna, not anymore. Eur J Cancer 2006;42:126–40.
- Azim HA, Jr., Peccatori FA. Treatment of cancer during pregnancy: The need for tailored strategies. J Clin Oncol 2010;28:e302–3; author reply e4.
- Azim HA, Jr., Peccatori FA, Pavlidis N. Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I: Solid tumors. Cancer Treat Rev 2010;36:101–9.
- Cardonick E, Dougherty R, Grana G, Gilmandyar D, Ghaffar S, Usmani A. Breast cancer during pregnancy: Maternal and fetal outcomes. Cancer J 2010;16:76–82.
- Amant F, Deckers S, Van Calsteren K, Loibl S, Halaska M, Brepoels L, . Breast cancer in pregnancy: Recommendations of an international consensus meeting. Eur J Cancer 2010;46:3158–68.
- Peccatori FA, Azim HA, Jr., Scarfone G, Gadducci A, Bonazzi C, Gentilini O, . Weekly epirubicin in the treatment of gestational breast cancer (GBC). Breast Cancer Res Treat 2009;115:591–4.
- Hahn KM, Johnson PH, Gordon N, Kuerer H, Middleton L, Ramirez M, . Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006;107:1219–26.
- Beadle BM, Woodward WA, Middleton LP, Tereffe W, Strom EA, Litton JK, . The impact of pregnancy on breast cancer outcomes in women < or = 35 years. Cancer 2009; 115:1174–84.
- Aziz S, Pervez S, Khan S, Siddiqui T, Kayani N, Israr M, . Case control study of novel prognostic markers and disease outcome in pregnancy/lactation-associated breast carcinoma. Pathol Res Pract 2003;199:15–21.
- Rodriguez AO, Chew H, Cress R, Xing G, McElvy S, Danielsen B, . Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol 2008;112:71–8.
- Moreira WB, Brandao EC, Soares AN, Lucena CE, Antunes CM. Prognosis for patients diagnosed with pregnancy-associated breast cancer: A paired case-control study. Sao Paulo Med J 2010;128:119–24.
- Guinee VF, Olsson H, Moller T, Hess KR, Taylor SH, Fahey T, . Effect of pregnancy on prognosis for young women with breast cancer. Lancet 1994;343:1587–9.
- Halaska MJ, Pentheroudakis G, Strnad P, Stankusova H, Chod J, Robova H, . Presentation, management and outcome of 32 patients with pregnancy-associated breast cancer: A matched controlled study. Breast J 2009; 15:461–7.
- Gentilini O, Cremonesi M, Toesca A, Colombo N, Peccatori F, Sironi R, . Sentinel lymph node biopsy in pregnant patients with breast cancer. Eur J Nucl Med Mol Imaging 2010;37:78–83.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
- Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, . Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol 2007;18:1632–40.
- Viale G, Regan MM, Mastropasqua MG, Maffini F, Maiorano E, Colleoni M, . Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst 2008;100:207–12.
- Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, . Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 2009;27:1168–76.
- Ishida T, Yokoe T, Kasumi F, Sakamoto G, Makita M, Tominaga T, . Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: Analysis of case-control study in Japan. Jpn J Cancer Res 1992;83:1143–9.
- Loibl S, Amant F, Kaufmann M, Ring A, Sileny H, Giermek J, . 313 patients with breast cancer during pregnancy – Results from a prospective and retrospective registry (GBG-20/BIG02-03). San Antonio Breast Cancer Symposium 2010; S6–2.
- Azim HA, Jr., Del Mastro L, Scarfone G, Peccatori FA. Treatment of breast cancer during pregnancy: Regimen selection, pregnancy monitoring and more. Breast 2011;20:1–6.
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, . In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003;17:1253–70.
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, . Control of mammary stem cell function by steroid hormone signalling. Nature 2010;465:798–802.
- Kim JB, Stein R, O'Hare MJ. Tumour-stromal interactions in breast cancer: The role of stroma in tumourigenesis. Tumour Biol 2005;26:173–85.
- Rouzier R, Werkoff G, Uzan C, Mir O, Gligorov J, Selleret L, . Pregnancy-associated breast cancer is as chemosensitive as non-pregnancy-associated breast cancer in the neoadjuvant setting. Ann Oncol 2011;22:1582–7.