Abstract
Background. In this modern era of multi-modality treatment there is increasing interest in the possibility of avoiding radical surgery in complete responders after neo-adjuvant long-course chemoradiotherapy (LCPRT). In this article, we present a systematic review of such treatments and discuss their therapeutic applicability for the future. Methods. We searched the PubMed online libraries to identify studies that reported on the long-term surgical and pathological outcomes after local excision together with those that explored the possibility of clinical observation only in patients achieving a complete clinical response after LCPRT. Results. Several retrospective (n = 10), one single-arm prospective, and one small randomised series have reported on the use of local excision after LCPRT and demonstrated acceptably low levels of local recurrence with survival comparable to patients progressing to conventional surgery. One prospective series allocated patients to observation or radical surgery based on histological parameters after local excision (ypT0 and ypT1) and showed no differences in outcomes. Two retrospective series from the same group on a Brazilian cohort of patients reported excellent long-term outcomes after “wait and watch” in complete clinical responders. However, other reports have shown no direct correlation between clinical and pathological response. Conclusion. Local excision may be an appropriate option for selected patients developing good clinical response after LCPRT. In our opinion, a policy of clinical observation in complete clinical responders after LCPRT may not be a safe strategy, unless we had robust predictive models for accurate identification of pathological complete response. In order to identify patients that may be potentially appropriate for such an approach we propose a clinical algorithm incorporating important clinical, radiological, and pathological parameters. The proposed model will require validation in a prospective study. Finally, we need randomised data for demonstrating the non-inferiority of clinical observation compared to conventional surgery before this can be considered as standard possible therapeutic option.
Surgical resection is the cornerstone of curative treatment for rectal cancer. The majority of patients have invasive tumours and conventionally these patients undergo radical excisional surgery. This invariably necessitates either an anterior resection (AR) with sphincter preservation, or an abdomino-perineal excision of rectum (APR) both being combined with total mesorectal excision (TME). Not surprisingly, these radical procedures are associated with considerable morbidity. In recent years there has been increasing interest in the role of multi-modality therapy to facilitate less radical surgery. Specifically, this relates to the role of downstaging long-course pre-operative chemo-radiotherapy (LCPRT). More recently, interest has developed around the possibility of avoiding surgical intervention altogether in patients who manifest a clinical complete response (cCR) after LCPRT.
In this article we review the evidence on the use of a multi-modality approach to promote less radical surgery in patients with rectal cancer and explore new technological developments in both radiology and molecular oncology that may act as useful adjuncts for appropriate identification of patients who may be considered for surgical conservation.
Methods
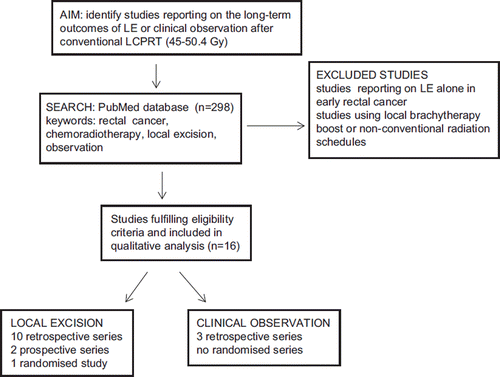
We searched the PubMeD online database using the following Keywords: rectal cancer, chemoradiotherapy, local surgery or observation. We identified studies which reported on the long-term pathological [tumour downstaging and pathological complete response (pCR)] and clinical [local recurrence (LR) and overall survival (OS)] outcomes of patients undergoing local excision (LE) or clinical observation alone after LCPRT. We selected studies that had employed conventional external beam radiotherapy schedules using 45–50.4 Gy in 1.8 Gy per fraction. We excluded studies that had reported on the outcomes of LE alone in early rectal tumours and those employing local brachytherapy boost following conventional radiotherapy, or non-conventional fractionation schedules.
The search revealed 298 citations of which we selected 10 retrospective studies and two prospective (single-arm) series that had reported on the long-term outcomes after local excision following LCPRT. One of these prospective series allocated patients to observation or radical surgery based on histological parameters after local excision. In addition, we identified one randomised trial of transanal-endoscopic microsurgery (TEMS) compared to TME.
We identified three retrospective studies which reported on the outcome of managing complete clinical responders after LCPRT with observation alone. We did not identify any prospective or randomised studies of standard surgery compared to observation alone after LCPRT. The methodology is schematically illustrated in .
Results
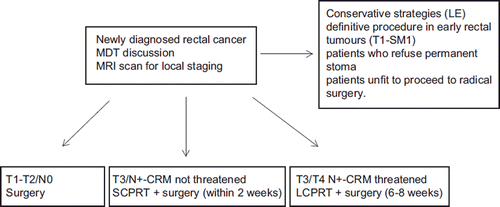
The management of rectal cancer in the UK is outlined in . Patients with very early T1 tumours with minimal sub-mucosal invasion may be considered for full-thickness LE as definitive procedure. However, patients with more advanced tumours are almost always advised radical surgery. Rarely patients developing excellent clinical response to LCPRT may be offered LE as an alternative less morbid local procedure if they refuse permanent stoma or are unfit to proceed to radical surgery. However, this approach is usually an exception and only after full explanation of the risks involved.
Figure 2. Management algorithm for patients with newly diagnosed rectal cancer in the UK. Conservative surgical strategies are employed only in few selected patients who out of personal choice do not want a permanent stoma or are not fit for radical surgery. LCPRT, long-course pre-operative chemoradiotherapy; MDT, multi-disciplinary team; SCPRT, short course pre-operative radiotherapy. LCPRT, long-couse pre-operative chemoradiotherapy.
The more frequent use of short-course pre-operative radiotherapy (SCPRT) in the UK and parts of Europe indicates that most patients are not appropriate for conservative surgical approach. Therefore, it is not surprising that most of the data on conservative surgical techniques originates from North America where most patients are treated with LCPRT including those with potentially operable disease without compromise of mesorectal fascia. Most trials of LCPRT have shown superior downstaging of primary tumour and lymph nodes compared to radiotherapy alone with 15–20% incidence of pCR, but without long-term survival benefit [Citation1–3].
Local surgical excision after chemoradiotherapy
The feasibility of performing full-thickness LE after neo-adjuvant LCPRT was initially evaluated as palliative protocol in patients not fit to proceed to radical surgery. One of the earliest series reported on the outcome of 14 patients with distal rectal tumours who were treated with full-thickness LE following high-dose radiotherapy. After median follow-up (FU) of 24 months the study reported three-year actuarial LR rate of 24% and OS of 61% [Citation4]. The same group published their experience on an expanded cohort of 48 patients who underwent LE six to eight weeks after completion of LCPRT. The study included patients with favourable tumour characteristics (T2; less than 3 cm) or higher tumour stage (T3; more than 3 cm) who developed complete or near-complete clinical response, and a separate cohort of poor-risk patients not fit for radical surgery. The study reported five-year OS of 84.5% and LR rate of 10% for the entire cohort. The levels of LR in patients developing good response to LCPRT were acceptably low (0 to 11%) and independent of tumour stage at presentation. In contrast, the group of surgically unfit patients whose tumours failed to downstage after LCPRT demonstrated a higher incidence of LR which approached 20% [Citation5].
Subsequently, several small retrospective series have reported on the long-term outcome of patients treated with LE following LCPRT. In one of the largest series from the M.D. Anderson Institute 47 patients with potentially operable rectal cancer [T3; N0 (n = 34), N1 (n = 13)] were treated with full-thickness LE after LCPRT. Twelve patients (25%) were considered to have prohibitive comorbidity and 15 patients (32%) refused to undergo TME. Fifteen patients (32%) developed cCR and LE was offered to them as an alternative treatment to TME after appropriate explanation that this was not considered the standard of care. Twenty-three (49%) patients had mural pCR and 17 (36%) had microscopic residual tumour. After median FU of 63 months 10 patients (22%) had developed disease recurrence [LR = 5; distant metastasis (DM) = 5] and the study reported 10-year OS of 74% which was comparable to the cohort of 473 patients treated with radical surgery (TME) [Citation6]. Similar results have been reported by other retrospective series which are summarised in [Citation7–13].
Table I. Published studies that have evaluated the effects of local excision (LE) after long-course pre-operative radiotherapy (LCPRT).
Borschitz et al. (2008) reported on a pooled analysis of patients treated with LE after LCPRT that included 273 patients from seven different studies. The study demonstrated that the probability of future LR was determined by the level of histological regression observed after LCPRT. None of the patients with pCR developed LR and ypT1 tumours were also associated with low rates of LR of 2%. In contrast, presence of ypT2 residual disease was associated with LR rates ranging from 6% to 20% which increased to 42% in patients with ypT3 disease [Citation14].
A large prospective series reported from an Italian group included 145 patients with T2 (n = 84) and T3 (n = 61) node-negative rectal cancer who underwent trans-anal endoscopic microsurgery (TEMS) after neo-adjuvant LCPRT. The definitive histology was ypT0 (17%), ypT1 (37%), ypT2 (34%), and ypT3 (12%). Eight patients (4%) with ypT2 (n = 5) or ypT3 (n = 3) tumours developed local recurrence. The rectal cancer-specific survival rate at the end of the follow-up period was 100% for ypT1, 90% for ypT2, and 77% for ypT3 patients [Citation15]. The same group reported on a phase 2 randomised study of 70 patients with good-prognosis T2-3 node-negative rectal cancer who were randomised to laparoscopic TME or TEMS following neo-adjuvant LCPRT. After median FU of 84 months no difference in LR was observed after TME (n = 2) or TEMS (n = 1). Similarly, the probability of cause-specific survival was 94% for TME and 94% for TEMS [Citation16].
More recently, the Polish Colorectal Study Group reported on an interim analysis of a prospective series of 47 patients with T1–3 node-negative rectal cancer who underwent LE after neo-adjuvant therapy (LCPRT or SCPRT followed by radiation boost) [Citation17]. According to the trial protocol patients with ypT0-1 tumours were managed expectantly compared to patients with ypT2/3 tumours with adverse prognostic features who proceeded to radical surgery. Twenty-nine (66%) patients had mural pCR or minimal residual ypT1 disease and were managed with close observation. Fifteen patients (34%) were advised to have radical surgery but only seven patients proceeded since the others were either unfit or refused operation. At 14 months of median FU only three patients (7%) in the observation group had developed LR and underwent successful salvage surgery.
Observation in complete clinical responders after chemoradiotherapy
An interesting concept to emerge in the recent past is whether surgery could be entirely avoided in patients developing a pCR after LCPRT which implies a favourable outcome. Patients developing pCR have extremely low rates of LR and may be more appropriate for a non-operative approach [Citation18]. However, there are no reliable pre-operative predictors of pCR and no randomised trials are available in which patients with a pCR to LCPRT were assigned to surgery or no surgery. Few investigators have evaluated the possibility of clinical observation in patients who develop clinical CR (cCR) after LCPRT. The above strategy was pioneered by a Brazilian group which reported encouraging results in a retrospective series of 265 patients with distal rectal tumours treated with LCPRT using 50.4 Gy in 28 fractions and concomitant 5-FU and leucovorin. Patients with an incomplete clinical response were referred for resection, while patients with a cCR [n = 71; T2 (n = 14); T3 (n = 49); T4 (n = 8)] were managed with clinical observation alone. Patients with cCR followed a rigid follow-up schedule which comprised of clinical assessments at one to two monthly intervals during the first 24 months using digital rectal examination (DRE), endoscopy, tumour markers and interval imaging (CT) at six monthly intervals. Patients were categorised as complete responders only after completion of 12 months of FU. Patients found to have pCR after surgical resection (n = 22) were compared directly with those in the observation group. With a median follow-up of 57 months, only two relapsed locally (both successfully salvaged) in the observation group and three developed metastatic disease. The five-year rates of OS and DFS were 100% and 92%, respectively. The corresponding values for the patients in the surgical group (median FU = 48 months) were 100% and 83%, respectively [Citation19,Citation20].
In an update of this study the group reported on an expanded cohort of 99 patients with cCR and demonstrated a five-year OS and DFS of 93% and 85%, respectively. After a mean FU of 49.9 months five (5%) endoluminal recurrences were detected which were successfully salvaged [Citation21]. Interpretation of the above results is limited by its retrospective nature and the lack of modern imaging techniques [particularly magnetic resonance imaging (MRI)] for evaluation of local disease.
In contrast to the above results, Hughes et al. (2010) reported on the outcome of 10 patients with rectal cancer who had a complete clinical response after LCPRT but could not proceed to surgery due to patient choice (n = 7) or medical co-morbidity (n = 3). Six of 10 patients died of recurrent disease and five (50%) patients developed local recurrence. The authors acknowledged the limitations of the small and retrospective nature of the report combined with the lack of a rigid follow-up schedule. However, they strongly argued against the uncritical extrapolation of “wait and watch” policy and cautioned against its use as a routine therapeutic option except in selected poor-risk surgical patients [Citation22].
Discussion
The local excision of rectal cancer has been employed as primary surgical procedure for patients with early rectal tumours (T1) limited to the mucosa and sub-mucosa. In early T1 tumours without high-risk features full thickness LE alone has been shown to be curative with long-term outcomes comparable to conventional radical surgery [Citation23]. Many techniques of LE have been described in the literature including the simple trans-anal, the posterior trans-sacral and trans-sphincteric procedures. However, the TEMS procedure represents the most sophisticated of these surgical techniques with several advantages including excellent exposure of the operative field which makes it easier to perform a complete full-thickness excision with an appropriate margin, and also to remove the adjacent perirectal fat using the same “holy” plane of dissection utilised in the TME.
Local surgical excision after chemoradiotherapy
There is no therapeutic role of performing local surgical excision on its own in rectal cancers extending into the muscularis propria (T2) or beyond – except as a purely palliative procedure. However, many studies have reported on the use of LE in patients developing good response after LCPRT and reported acceptably low rates of LR and long-term survival comparable to patients proceeding to conventional radical surgery. The encouraging results from above studies have renewed interest in the possibility of avoiding radical surgery in selected sub-group of patients following neo-adjuvant CRT. However, the interpretation of above data is confounded by the predominant retrospective nature of the studies. Moreover, these studies cannot be directly compared due to the presence of significant heterogeneity with respect to patient and tumour characteristics resulting from the lack of consistent staging and selection criteria. For example, some patients were likely to have been considered for LE at the outset whereas others may have been selected for LE after developing good clinical response to CRT. These disease subsets are likely to manifest different biological behaviour and patterns of recurrence. The studies are also not balanced with respect to tumour characteristics with some studies including predominantly early rectal tumours (T1/T2). Furthermore, the lack of consistent staging criteria would render it difficult to collectively compare the outcome of individual tumour stages across the studies. More importantly, the extent and quality of local surgery is likely to have significantly varied between studies depending on the individual technique and the skills of the surgeon involved.
Patients with minimal residue disease (ypT1) following LE are usually managed with observation, but patients with ypT2 or higher tumours are offered radical surgery. Indeed, some investigators have proposed the presence of minimal residual disease (ypT1) as a satisfactory outcome after full-thickness LE [Citation14,Citation17]. However, the potential caveat of using mural response as the only criteria for selecting patients for LE was highlighted in a retrospective study of 242 patients following LCPRT. The incidence of lymph node involvement was 3.2% in patients developing mural pCR (ypT0) compared to 11% with ypT1 tumours and increased further as the ypT stage increased (ypT2 = 29.2%; ypT3 = 37.3%). The authors argued against the policy of limited surgery in patients with ypT1 residual tumour and considered this to be a possible option only in patients developing mural pCR [Citation24].
Patients considered for possible LE should have their tumours tattooed prior to commencing LCPRT to facilitate full-thickness LE at site of primary tumour. The above was highlighted in the Polish study in which three of four patients with lateral margin involvement after LE had no visible tattoos at the time of surgery to guide surgical excision. Previous series have shown that up to 10% of patients with cCR may have no visible mucosal abnormality to target local excision [Citation25].
The possibility of surgical salvage is considered for all fit patients developing LR after previous limited surgical procedures. However, the type of salvage surgery may be influenced by other factors including the functional and structural integrity of the rectal wall after previous LCPRT and LE. In the Polish series three patients underwent salvage surgery with APR despite being considered appropriate for sphincter preservation at presentation [Citation17].
Observation in complete clinical responders after chemoradiotherapy
Data from Brazilian series demonstrated excellent long-term local control and OS rates in patients developing cCR after LCPRT. However, it is well documented that patients with cCR may still harbour residual disease. The limitations of clinical assessment after LCPRT were demonstrated in a prospective series of 94 patients who underwent assessment with DRE and sigmoidoscopy prior to and after completion of LCPRT. Clinical assessment underestimated pathologic response in 73 patients and DRE was able to identify only three of 14 cases (21%) with a pCR. The overall concordance between clinical evaluation and actual pathologic response was only 22% [Citation26]. In another retrospective review of 488 patients with rectal cancer following LCPRT the cCR rate for the entire cohort was 19%, but only 10% had a true pCR [Citation27].
Therefore, it is not surprising that the Brazilian experience has generated intense debate with some investigators expressing concerns about employing a policy of watchful expectancy based entirely on the presence of cCR after LCPRT [Citation22,Citation28]. In patients being considered for observation after cCR the extent of local disease at presentation is likely to be an important determinant of long-term outcome. In the Brazilian series approximately 20% of the original 265 patients had T2N0 disease which may have influenced the favourable outcome.
When should response be assessed?
The optimal time interval for assessing the response after LCPRT remains controversial. The current standard of care is to operate six to eight weeks after the completion of CRT, but more recent data support the use of prolonged elective delay for facilitating tumour downstaging. However, prolonged time-interval prior to surgery raises legitimate concerns about the possibility of inferior outcomes from tumour repopulation unless pCR was achieved. Kalady et al. (2009) reported on retrospective analysis of 242 patients with rectal cancer treated with LCPRT which included 58 (24%) patients with pCR. The authors identified the waiting time of more than eight weeks as the only predictor of pCR on multivariate analysis [Citation29]. Habr-Gama et al. (2008) reported on a Brazilian series of 250 patients who underwent surgery at less (n = 121), or more (n = 129) than 12 weeks after LCPRT [Citation30]. Patients in the delayed surgical group had significantly earlier disease stage (p = 0.009) and decreased risk of lymph node metastases (p = 0.015) and the survival outcomes were similar in the two groups. In a recent prospective phase II study patients undergoing delayed (11 weeks) surgery had superior pCR rate (25% versus 18%) compared to group having surgery at six weeks after LCPRT [Citation31]. The optimum time-interval prior to surgery can be only defined following availability of mature data from prospectively randomised studies.
Novel imaging protocols for prediction of response after chemoradiotherapy
In the last decade an interesting area of research has revolved around the use of novel imaging modalities – FDG-PET and diffusion-weighted MRI (DW-MRI) scans – to predict the probability of response after LCPRT. Although conventional MRI scans are invaluable tools for the pre-operative staging of rectal tumours, they have inherent limitations in assessing the response to LCPRT. For example, the treatment may induce changes in the tumour micro-environment which effectively decreases tumour viability without producing significant change in tumour size [Citation32]. Furthermore, MRI scan cannot reliably differentiate CRT-induced scarring from residual disease [Citation33]. In a prospective study MRI scan was reported to have PPV of 85% and NPV of 67% for response assessment after CRT. It can be particularly difficult to detect islands of residual tumour cells within areas of radiation-induced fibrosis [Citation34].
Imaging using DW-MRI is an interesting novel technology which is sensitive to the tissues biophysical characteristics, including apparent diffusion coefficients (ADCs) and volume fractions of water in different populations. Pre-treatment values of ADC reflecting tissue viability based on water diffusion characteristics have been shown to correlate significantly with probability of tumour response to radiation [Citation35]. This correlation implies that tumours with low pre-treatment diffusion values, indicating high viability, will respond better to radiotherapy than tumours with high diffusion values which indicate the presence of mucin or necrosis. Preliminary results indicate that diffusion-weighted MRI may be an effective diagnostic strategic tool for predicting the outcome of patients treated with LCPRT. Recently, Sun et al. (2010) reported on the probability of using the pattern of ADC modulation during the first two weeks of CRT as a predictive indicator of tumour-related response. In a prospective study of 37 patients treated with LCPRT they showed that responding tumours had a lower mean tumour ADC at presentation compared to the tumours which failed to respond to treatment. At the end of the first week of CRT, the mean tumour ADC increased significantly in the downstaged group, compared to the radioresistant group in whom the ADC was relatively unchanged [Citation36].
The limitations of conventional imaging have also generated considerable interest in the possible role of molecular imaging such as FDG-PET to identify potential responders to LCPRT. However, PET assessment is likely to be influenced by non-malignant metabolic processes including treatment-induced inflammation. Therefore, the exact timing of PET imaging combined with the selection of robust specific parameters to assess response is of paramount importance. Several studies have evaluated the use of PET in rectal cancer to predict the response to CRT using various parameters, including SUVmean, SUVmax, PET-derived tumour size, visual response score, and change in total lesion glycolysis. Capirici et al. (2006) reported on one of the largest series of 88 patients with rectal cancer who underwent PET imaging six weeks after the completion of CRT. The PET images were evaluated based on a range of pre-defined SUVmax values combined with visual score in patients with an equivocal SUV result. After a median follow-up of 38 months, the OS was 91% in patients with negative post-treatment PET and 72% in those with a positive PET (p = 0.024) [Citation37]. In a subsequent report on PET outcome in 44 patients the response index (RI) representing the absolute and percent SUVmax difference between pre- and post-CRT PET scans was shown to be useful predictive marker that correlated with histological regression. Based on a pre-defined threshold estimate the investigators demonstrated an overall 80% accuracy of PET in predicting response to CRT with 79% PPV and 89% negative predictive value [Citation38]. Other studies have reported similar results [Citation39–42].
More recently, interesting data has emerged on combining the two different imaging modalities for determining the probability of pCR after LCPRT. Lambrecht et al. (2010) reported on a study of 22 patients with rectal cancer treated with LCPRT. These patients underwent FDG-PET at the start of CRT, after 10 to 12 fractions, and five weeks after the end of treatment. Patients also underwent DW-MRI before the start of CRT with determination of mean ADCs. The difference in SUVmax at the start and during or after CRT [ΔSUV (max)] combined with the initial ADC values correlated significantly with the pathological response to treatment. The receiver operating characteristics (ROC) curve analysis revealed an optimal threshold for ΔSUV (max) of 40% during CRT and 76% after CRT for predicting the probability of pCR. The authors postulated the existence of a predictive algorithm for pCR with a sensitivity of 100% and specificity of 94% by combining the optimal ΔSUV (max) thresholds during and after CRT, or by combining the thresholds for the initial ADC value and the ΔSUV (max) during CRT [Citation43].
Molecular markers of radiosensitivity
Most traditional assays of radiosensitivity involve the plotting of cell survival curves following exposure of in vitro tumour cell lines to range of radiation doses. The clonogenic assay is the most commonly employed marker of radiosensitivity. However, it is well known that in vitro cell culture does not reflect the tumour microenvironment as it is devoid of the spatial cellular organisation and cell-matrix interactions characteristic of tumour tissue. The 3D-spheroid culture model was developed to make the cellular environment more representative of in vivo tissue, but this too is widely acknowledged to not truly reflect the natural or pathophysiological state.
More exciting has been the development of novel research methodologies involving microfluidic technology to provide real-time in vitro assessment of tissue biopsies. Lab on a chip or micro-total analysis systems (mTAS) provide a way of maintaining small clinical biopsies (2–3 mm3) of tissue for up to eight days. Drugs or other potential therapeutic interventions, such as radiotherapy can be applied to the tissue and the effects monitored downstream through inclusion of analysis modules on the microfluidic device [Citation44,Citation45]. Recently our group has demonstrated, in a proof of concept study, that human colorectal tissue biopsies and normal mucosal tissue can be subjected to hypoxic episodes causing the release of vascular endothelial growth factor into the media from the tumour but not the normal biopsy. The result shows that the tissue behaved as predicted, i.e. the malignant tissue had up-regulated intracellular pathways involved in angiogenesis that would not be present in the normal biopsy [Citation46]. The simple and flexible nature of the described devices makes them ideal tools to study exposure of differing chemotherapy drugs and radiation doses. A forthcoming study will assess the robustness of the model in predicting radiosensitivity by direct validation of the in vitro results against pathological regression observed after CRT.
Possible futuristic algorithm to select patients in whom radical surgery could be avoided
In our opinion, the decision to adopt a policy of “wait and watch” based entirely on cCR could be unsafe and associated with considerable risks. The possibility of LE may be considered in selected patients developing good clinical response after LCPRT in whom quality of life takes precedence over the small and unpredictable probability of higher long-term LR. Clearly, patients need to be fully informed as the risks of such an approach.
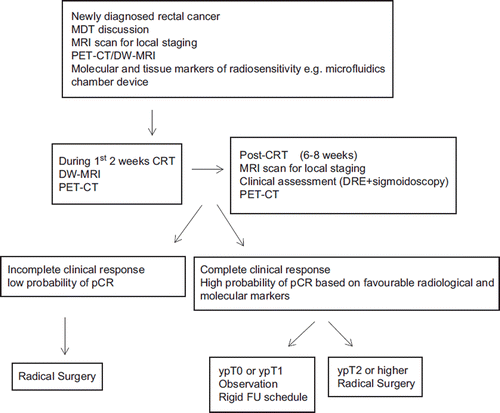
Indeed, the wider and long-term applicability of using above therapeutic strategies as a viable treatment option in the management of rectal cancer is primarily dependent on the development of robust predictive models for reliable evaluation of the probability of tumour response and pCR after LCPRT. In our opinion such predictive models should incorporate pathological, radiological and molecular markers and provides a schematic illustration of a potential futuristic clinical algorithm that may be employed to select appropriate patients in whom radical surgery can be avoided. The proposed algorithm incorporates diagnostic modalities PET/CT and DW-MRI which have been shown to have high sensitivity and specificity for the prediction of pCR after LCPRT. However, the feasibility of using such diagnostic protocol in routine clinical practice will require prospective validation, including a comprehensive cost-effective analysis which should incorporate quality-adjusted life year (QALY) measures. Because of the devastating consequences which may arise from a probable error in clinical judgement it is unlikely we will be able to perform a direct randomised comparison of conservative approach versus standard radical surgery without the inclusion of efficient “checkpoints” using the above predictive models. Finally, we will need data from randomised studies designed specifically to assess the non-inferiority of conservative approach using local recurrence and OS as primary end-points and quality of life (QOL) as a secondary end-point before we can offer the option of surgical conservation routinely to our patients. In the absence of such data conservative surgery after LCPRT is likely to remain a second-choice therapeutic option for patients who are elderly and frail and not fit to proceed to conventional surgery.
Figure 3. Schematic illustration of probable futuristic clinical algorithm for patients considered for conservative management after long-course pre-operative radiotherapy (LCPRT). The paradigm illustrates various molecular, radiological and histological parameters that may be employed at different time-intervals during patient's treatment pathway for the reliable pre-operative identification of pathological complete response (pCR).
Conclusion
In summary, therefore, there is increasing evidence to suggest that a minority of patients following LCPRT may be able to avoid radical surgical resection. However, in the absence of accurate predictive markers a non-operative policy remains difficult to justify at present. In our opinion, these strategies should be still considered experimental in nature and only offered to selected patients who cannot proceed to radical surgery due to poor fitness, or out of personal choice. Ideally, these patients should be entered into a clinical trial. The development of powerful predictive models in the future may facilitate the possibility of limited surgical intervention in patients with high probability of pCR. We have outlined the clinical paradigm that may be applicable to these patients and future randomised studies evaluating the effects of surgical conservation compared to standard radical surgery should include similar predictive models within their respective protocols.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009; CD006041.
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–23.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, . EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. New Eng J Med 2006;355:1114–23.
- Marks G, Mohiuddin MM, Masoni L, Pecchioli L. High-dose preoperative radiation and full-thickness local excision. A new option for patients with select cancers of the rectum. Dis Colon Rectum 1990;33:735–9.
- Mohiuddin M, Marks G, Bannon J. High-dose preoperative radiation and full thickness local excision: A new option for selected T3 distal rectal cancers. Int J Radiat Oncol Biol Phys 1994;30:845–9.
- Callender GG, Das P, Rodriguez-Bigas MA, Skibber JM, Crane CH, Krishnan S, . Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol 2010;17:441.
- Kundel Y, Brenner R, Purim O, Peled N, Idelevich E, Fenig E, . Isocal excision after complete pathological response to neoadjuvant chemoradiation for rectal cancer an acceptable treatment option?. Dis Colon Rectum 2010; 53:1624–31.
- Schell SR, Zlotecki RA, Mendenhall WM, Marsh RW Vauthey JN, Copeland EM 3rd., . Transanal excision of locally advanced rectal cancers downstaged using neoadjuvant chemoradiotherapy. J Am Coll Surg 2002;194:584.
- Ruo L, Guillem JG, Minsky BD, Quan SH, Paty PB, Cohen AM. Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorectal Dis 2002;17:54.
- Kim CJ, Yeatman TJ, Coppola D, Trotti A, Williams B, Barthel JS, . Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg 2001;234:352.
- Bonnen M, Crane C, Vauthey JN, Skibber J, Delclos ME, Rodriguez-Bigas M, . Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys 2004; 60:1098.
- Huh JW, Jung EJ, Park YA, Lee KY, Sohn SK. Preoperative chemoradiation followed by transanal excision for rectal cancer. J Surg Res 2008;148:244–50.
- Nair RM, Siegel EM, Chen DT, Fulp WJ, Yeatman TJ, Malafa MP, . Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg 2008;12:1797–805; discussion 1805–6. Epub 2008 Aug 15.
- Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol 2008;15:712.
- Guerrieri M, Baldarelli M, Organetti L, Grillo Ruggeri F, Mantello G, Bartolacci S, . Transanal endoscopic microsurgery for the treatment of selected patients with distal rectal cancer: 15 years experience. Surg Endosc 2008; 22:2030–5.
- Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S, . A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 2008;22:352–8.
- Bujko K, Richter P, Kołodziejczyk M, Nowacki MP, Kulig J, Popiela T, . Polish Colorectal Study Group. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders. Radiother Oncol 2009;92:195–201. Epub 2009 Mar 16.
- de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Geisler D, Dietz DW, Lavery IC, . Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol Epub 2011 Jan 5.
- Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, . Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg 2004;240:711.
- Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis 2006;8(Suppl 3):21.
- Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, . Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006;10:1319–28; discussion 1328–9.
- Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy?. Acta Oncol 2010;49:378–81.
- Winde G, Nottberg H, Keller R, Schmid KW, Bünte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum 1996;39:969–76.
- Mignanelli ED, de Campos-Lobato LF, Stocchi L, Lavery IC, Dietz DW. Downstaging after chemoradiotherapy for locally advanced rectal cancer: Is there more (tumor) than meets the eye?. Dis Colon Rectum 2010;53:251–6.
- Bedrosian I, Rodriquez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, . Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg 2004;8:56–63.
- Guillem J, Chessin D, Shia J, Moore HG, Mazumdar M, Bernard B, . Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol 2005;23:3475–9.
- Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, . Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: An analysis of 488 patients. J Am Coll Surg 2002;194:131.
- dos Santos LV, dos Anjos Jácome AA, Cárcano FM, da Silveira Nogueira Lima JP, Serrano SV. Watch and wait policy remains experimental for the management of rectal cancer. Colorectal Dis 2010;12:833.
- Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, . Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg Epub 2009 Aug 25.
- Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, . Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome?. Int J Radiat Oncol Biol Phys 2008;71:1181–8.
- Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97–102.
- Hopkins S, Fakih M, Yang GY. Positron emission tomography as predictor of rectal cancer response during or following neoadjuvant chemoradiation. World J Gastrointest Oncol 2010;2:213–7.
- Capirci C, Rubello D, Chierichetti F, Crepaldi G, Carpi A, Nicolini A, . Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: Role of F18-FDG PET. Biomed Pharmacother 2004;58:451–7.
- Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, . Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009;250:730–9.
- Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, . Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia 2004;6:136–42.
- Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, . Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: Preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 2010;254:170–8. Epub 2009 Dec 17.
- Capirci C, Rubello D, Chierichetti F, Crepaldi G, Fanti S, Mandoliti G, . Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. AJR Am J Roentgenol 2006;187:W202–8.
- Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, . Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging 2007;34:1583–93.
- Amthauer H, Denecke T, Rau B, Hildebrandt B, Hünerbein M, Ruf J, . Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: Correlation with endorectal ultrasound and histopathology. Eur J Nucl Med Mol Imaging 2004;31:811–9.
- Rosenberg R, Herrmann K, Gertler R, Künzli B, Essler M, Lordick F, . The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis 2009; 24:191–200.
- Konski A, Li T, Sigurdson E, Cohen SJ, Small W Jr, Spies S, . Use of molecular imaging to predict clinical outcome in patients with rectal cancer after preoperative chemotherapy and radiation. Int J Radiat Oncol Biol Phys 2009;74:55–9.
- Janssen MH, Ollers MC, van Stiphout RG, Riedl RG, van den Bogaard J, Buijsen J, . PET-based treatment response evaluation in rectal cancer: Prediction and validation. Int J Radiat Oncol Biol Phys Epub 2011 Mar 5.
- Lambrecht M, Deroose C, Roels S, Vandecaveye V, Penninckx F, Sagaert X, . The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol 2010;49:956–63.
- Wlodkowic D, Cooper JM. Tumors on chips; oncology meets microfluidics. Curr Op Chem Biol 2010;14:1–12.
- Webster A, Greenman J, Haswell SJ. Development of microfluidic devices for biomedical and clinical application. J Chem Technol Biotechnol 2010. DOI 10.1002/jctb.2482.
- Webster A, Dyer CE, Haswell SJ, Greenman J. A microfluidic device for tissue biopsy culture and interrogation. Anal Methods 2010;2:1005–7.