Abstract
Background. Fast and accurate work-up is crucial to ensure the best possible treatment and prognosis for patients with head and neck cancer. The presence or absence of neck lymph node metastases is important for the prognosis and the choice of treatment. Clinical lymph node (N)-staging is done by palpation and diagnostic imaging of the neck. We investigated the current practice of the initial radiological work-up of patients with oral squamous cell carcinomas (OSCC) in the Nordic countries. Methods. A questionnaire regarding the availability and use of guidelines and imaging modalities for radiological N-staging in OSCC was distributed to 21 Head and Neck centres in Denmark (n = 4), Finland (n = 5), Iceland (n = 1), Norway (n = 4) and Sweden (n = 7). We also asked for a description of the radiological criteria for determining the lymph nodes as clinical positive (cN+) or negative (cN0). Results. All 21 Head and Neck centres responded to the questionnaire. Denmark and Finland have national guidelines, while Norway and Sweden have local or regional guidelines. Seventeen of the 19 centres with available guidelines recommended computed tomography (CT) of the cN0 neck. The waiting time may influence the imaging modalities used. Lymph node size was the most commonly used criteria for radiological cN+, but the cut-off measures vary from 0.8 to 2.0 cm. Conclusion. Overall, CT is the most commonly recommended and used imaging modality for OSCC. Despite availability of national guidelines the type and number of radiological examinations vary between centres within a country, but the implementation of a fast-track programme may facilitate fast access to imaging. The absence of uniform criteria for determining the lymph nodes of the neck as cN+ complicates the comparison of the accuracy of the imaging modalities. Well-defined radiological strategies and criteria are needed to optimise the radiological work-up in OSCC.
Oral cavity cancer is the eleventh most common cancer worldwide. The absolute incidence of oral cavity cancer was 1262 cases in 2008 in the Nordic countries; Denmark, Finland, Iceland, Norway and Sweden [Citation1]. Oral squamous cell carcinomas (OSCC) constitute approximately 95% of oral cavity cancers [Citation2]. OSCC predominantly spreads to the regional lymph nodes and the presence of metastatic lymph nodes is the single most important prognostic factor for survival [Citation3].
OSCC is staged according to the Tumour-Nodes-Metastases (TNM) classification of the Union for International Cancer Control [Citation4]. Depending on the clinical (i.e. pretreatment) TNM classification, the resectability of the tumour and operability of the patient, treatment with surgery or radiotherapy or both is advised. The initial clinical N-staging (cN-staging) of OSCC concerns the lymph nodes of the neck and is determined by palpation of the neck in combination with diagnostic imaging, such as ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography-CT (PET-CT). The rationale for using imaging is that palpation of the neck has a lower sensitivity compared to imaging, e.g. CT [Citation5]. Accordingly, in the “Clinical Practice Guidelines in Oncology; Head and Neck Cancers” by The American National Comprehensive Cancer Network, CT and/or MRI of the primary tumour as well as of the neck is recommended in the work-up of oral cavity cancer. However, the guidelines do not describe a prioritisation of the imaging modality, nor do they describe the radiological criteria for determining the lymph nodes as metastatic (N+). Besides recommendations, other factors such as tradition, availability of imaging modality, and structure of national health care systems may influence on the radiological work-up of cN-staging of OSCC.
The Nordic countries constitute a population of approximately 25.1 million inhabitants [Citation6] and have similar structure in their national health care system [Citation7]. All patients have free access to treatment of oral cavity cancer, which is centralised at multidisciplinary Head and Neck (H&N) centres. A surgeon initially examines the patients with suspected OSCC and decides what kind of imaging should be performed. The results of the physical examination, imaging and biopsy are evaluated at a multidisciplinary board meeting in order to clinically stage the tumour. Based on all this information the treatment strategy is decided.
The aim of the present study was to investigate and compare the initial radiological work-up, which supplements the initial clinical examination prior to the clinical staging of the neck and the decision on treatment of OSCC within the Nordic countries. Specifically, we addressed the availability of clinical guidelines and the recommendations for imaging modality provided in the guidelines. Moreover, the access to different imaging examinations, the current use of imaging, and the radiological criteria for N-staging of the neck were addressed.
Materials and methods
We conducted a multicentre questionnaire study on the initial radiological N-staging of the neck in OSCC at the Head and Neck (H&N) centres in the Nordic countries.
Between May and December 2010 we corresponded with the 21 H&N centres, identified by the board of Scandinavian Society for Head and Neck Oncology, as being responsible for the diagnostic work-up and treatment of oral cavity cancer. The centres were located in: Denmark (Odense, Aarhus, Aalborg and Copenhagen) with 5.5 million inhabitants; Finland (Oulu, Tampere, Turku, Kuopio, Helsinki) with 5.3 million inhabitants; Iceland (Reykjavik) with 0.3 million inhabitants; Norway (Oslo, Trondheim, Tromsø and Bergen) with 4.8 million inhabitants; and Sweden (Göteborg, Örebro, Uppsala, Linköping, Umeå, Stockholm and Lund) with 9.2 million inhabitants. The average number of inhabitants per centre is highest in Denmark with 1.4 million inhabitants per centre, and lowest in Iceland, with 0.3 million inhabitants per centre. The senior clinician in charge of oral cavity cancer treatment from each centre was invited to participate in the study. Each senior clinician was responsible for depicting the clinical practices at their respective H&N centre by means of a questionnaire and urged to contribute to the discussion of the outcome for the study. The clinician representing their respective H&N centre comprised head-and-neck surgeons, oncologists, plastic surgeons, radiologists and other specialities involved in the work-up and treatment of OSSC. Specifications of contributors are presented in the authorship list.
The questionnaire primarily addressed the imaging availability and usage, but also the availability of local-, regional- or national clinical guidelines on the work-up and treatment of oral cavity cancer was requested. The clinicians were asked to answer Yes or No to the availability of the following imaging modalities at their own or at a collaborating hospital: US, CT, MRI or PET-CT. The time from referral to the imaging examination (waiting time) was assessed by ticking off one of the following intervals: 0–2, 3–7, 8–14, 15–30 or 30+ days. Questions about the diagnostic work-up were asked separately for the palpable lymph node negative (N0) neck and for clinical lymph node positive neck (N+). If guidelines included recommendations for imaging of the neck, clinicians were asked to specify the recommendations for CT, MRI, diffusion weighted MRI (dwMRI), US, Doppler US, contrast enhanced US or PET-CT. Moreover, the percentage of patients (0–20%, 20–40%, 40–60%, 60–80%, 80–100%) examined with each modality was estimated. Finally, clinicians were asked to describe the radiological criteria used for determining lymph nodes as N+. Data were collected for descriptive analysis. The questionnaire is available as an online attachment http//www.informahealthcare.com/doi/abs/ 10.3109/0284186X.2011.640346.
Results
All of the 21 H&N centres responded to the invitation. Of the 21 senior clinicians representing each centre, 19 were ENT surgeons, one was plastic surgeon and one was oncologist.
Availability of guidelines
National guidelines were available in Finland and Denmark. The seven centres in Sweden had regional and/or local guidelines. In Norway two of the four centres had regional or local guidelines and one centre referred to the Danish guidelines. Iceland and one centre in Norway had no guidelines, consequently 19 H&N centres had guidelines. The Finnish guidelines are updated annually by the Finnish Head and Neck Oncology Group. According to the Finnish guidelines either CT or MRI should be used, and US is used to obtain fine needle aspiration cytology (FNAC) from the lymph nodes of the neck.
The Danish guidelines (Cancer pathways) were published by the National Board of Health in 2008 and are based on the national guidelines created by the Danish Head and Neck Cancer Group [Citation8]. According to the Cancer pathways imaging with CT and MRI are recommended for the primary tumour (T-site), and also for the neck. In addition, this may be supplemented with US of the neck.
Availability of the imaging modalities
Nineteen centres had access to all imaging modalities (Conventional US, CT, MR and PET-CT) at their own hospital. Two centres had access to PET-CT at other hospitals.
Waiting times
The waiting time is defined as the estimated number of days from referral to radiological examination. The median waiting time was 3–7 days for US and CT and 8–14 days for MRI and PET-CT. The waiting time did not differ substantially between countries, but varied (from 0 to 30 days) between centres within countries.
Guideline recommendations
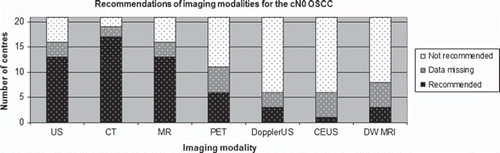
The number of H&N centres recommending a given imaging modality for OSCC with clinical lymph node negative neck (cN0) determined by palpation of the neck is shown in .
Figure 1. Recommendation of imaging for the cN0 OSCC as sum of H&N centres. CEUS, contrast enhanced ultrasound; DWMRI, diffusion weighted magnetic resonance imaging.
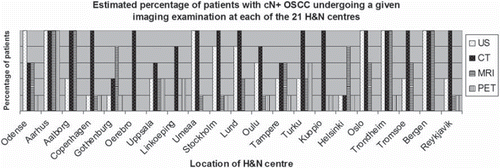
The recommendations for imaging modalities for OSCC with clinical lymph node positive neck (cN+) are illustrated in .
Figure 2. Recommendation of imaging for the cN+ OSCC as sum of H&N centres. CEUS, contrast enhanced ultrasound; DWMRI, diffusion weighted magnetic resonance imaging.
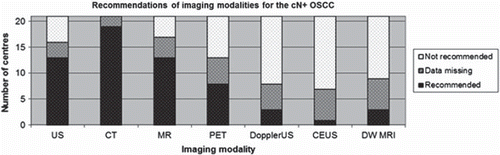
Seventeen of the 19 H&N centres with available guidelines recommended CT of the cN0 neck, while in the case of the cN+ all 19 centres recommended CT. US were recommended at 13 centres for both cN0 and cN+, but only at one centre was it the sole imaging modality of choice. MRI was recommended at 13 centres for both cN0 and cN+. PET-CT was recommended by six centres for the cN0 neck and by eight centres for the cN+ neck. Contrast enhanced US, Doppler US and dwMRI were only recommended at one, two and three H&N centres respectively and have not been included in the and .
Imaging examinations performed
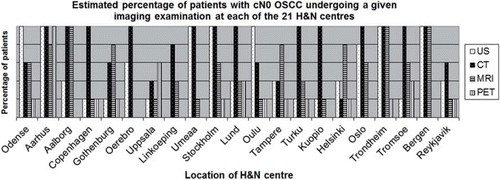
For each imaging modality the percentage of patients undergoing the given imaging examination was estimated. shows the estimated percentage of patients with cN0 undergoing US, CT, MRI and PET-CT examination at each of the 21 H&N centres.
At 13 of the 21 centres the majority of patients (80–100%) with cN0 underwent CT. The second most used imaging modality was US (7 of 20 centres). MRI was used in 60–80% of the patients at four centres and in 80–100% at three centres. PET-CT was used for 0–20% of the patients at 10 centres and was used for selected cases such as large tumours, late stage (T4), and when distant metastases were suspected. Concerning differences between imaging of cN0 and cN+ ( and ) there were no uniform alteration in the utilisation of US, CT, MRI and PET-CT. The accumulated number of imaging examinations performed at each centre varied, and some patients underwent more than one imaging examination.
Malignancy criteria
The radiological criteria for determining the lymph node as positive varied between centres and one centre did not describe the criteria employed. Responders representing the H&N centre including the radiological department provided the specifications of the radiological criteria.
The size of the lymph node was the most widely used criterion for CT, MRI and US (19 centres). The cut-off measures were identical for CT and MRI, while for US one centre reported a lower cut-off then for CT and MRI. The cut-off measurement for the short axis varied from 0.8 to 1.5 cm and from 1.0 to 2.0 cm for the long axis. Five centres differentiated between the sizes depending of the location on the neck, resulting in a slightly larger cut-off measure for the jugulodigastric lymph nodes compared to lymph nodes at the rest of the neck (e.g. 0.8 vs. 1.1 cm). A ratio, of the longitudinal diameter to the short axis diameter, less than 2 was used as a malignant criterion in three centres. Moreover, morphological description such as: roundness (eight centres); border irregularities (four centres); and central necrosis or irregular contrast enhancement (10 centres) were used for CT, MRI and US. Additional criteria for US were hypoechoic appearance (two centres), absence of a fatty hilum (three centres) and increased peripheral blood (three centres) flow detected by colour Doppler. One centre described the criteria for PET as: Any focal tracer uptake beyond background in lymph node located in region where (neck) metastasis can be expected.
Discussion
In this multicentre questionnaire survey we found that recommendations and utilisation of imaging of the neck in OSCC differed between and within the Nordic countries. Moreover, the radiological criteria for determining lymph nodes as metastatic were not uniform. Overall, CT was the most commonly recommended and used imaging modality. According to guidelines US and MRI were evenly recommended, but in the clinical setting US was the second most used imaging, while MRI is the third most used modality. This may be due to the longer waiting time for MRI and/or the higher cost for MRI compared to CT or US. Thus, availability of guidelines does not per se ensure fast access to the recommended imaging.
N-staging is important since the treatment of the neck may differ between cN0 and cN+. Furthermore, over the past decades the number of imaging examinations in head and neck cancers has increased [Citation9] and rendering some unresolved issues.
First, the availability of imaging is very high in the Nordic countries and this study confirms that it is the waiting time rather than the absence of a given imaging modality, which limits the choice of radiological workup. In the present study, four centres reported that 80–100% of the N+ patients underwent MRI. At these four centres MRI was performed within seven days, suggesting that fast access is an important factor in the choice of imaging modality. Concerning the waiting times for US, the examinations are performed within two days at seven centres and five of these centres reported that the surgeon performs the US. This indicates a reduction of waiting time when US is performed by the surgeon versus the radiologist. However, US is very operator dependent and minimum training recommendations for the head and neck US equal to other “organ-specific” guidelines from the European Federation of Societies for Ultrasound in Medicine and Biology, would be desirable. Also of importance is that an increased interval between diagnosis and treatment has been shown to correlate positively with the risk of a progressive course of head and neck cancers [Citation10,Citation11]. Nevertheless, two Nordic studies have experienced increasing waiting time from diagnosis to treatment of head and neck cancers during the 1990s [Citation9,Citation12]. Interestingly, a recent Danish study has indicated that a focused effort to reduce waiting time can reduce the delay from referral to treatment for head and neck cancer by around 50% [Citation13]. In Denmark, the effort to reduce waiting time is addressed by means of a fast track programme for all cancer diagnoses. This programme imposes that when cancer is clinically suspected at an ENT centre, the clinical work-up must be terminated within seven days [Citation14]. Consequently, the waiting time for CT in Denmark is limited to seven days. In Sweden, data about patients with OSCC have been registered in the Swedish Head and Neck Cancer Registry since 2008, thereby the waiting time between referral and treatment can be continuously evaluated. The ultimate endpoint in an evaluation of the radiological imaging of patients with OSCC is improved survival. However, improvement in survival rates cannot be directly inferred from radiological work-up, but accurate imaging may reduce morbidity in terms of stratifying patients for tailor-made treatment. Second, the results of the present study raise the question of which imaging examinations should be performed. The literature on imaging in head and neck cancer is extensive, but the variation of the recommendations and use of imaging shown in this study, may reflect the low level of evidence for imaging of OSCC. A recent meta-analysis evaluated the detection of lymph node metastases in head and neck cancer for different imaging modalities. The meta-analysis showed that US guided fine needle aspiration cytology (FNAC) has the highest accuracy (calculated as a diagnostic odds ratio) followed by US, CT and MRI [Citation15]. One weakness of the meta-analysis is the different criteria for determination of lymph node metastases; a notion which is also apparent in the present study. The size of the cut-off value for discriminating between benign and metastatic lymph node is arbitrary, since it is a trade-off between sensitivity and specificity [Citation16].
Furthermore, the meta-analysis does not include PET-CT. Several studies have been published on the N-staging of head and neck cancer with PET-CT, and the results are not consistent. Some studies have showed a higher rate of detection with PET-CT than CT or MRI [Citation17,Citation18] whereas others have not [Citation19–21]. This equivocal advantage of PET–CT for initial N-staging of head and neck cancer is reflected in the imaging performed in the Nordic countries, where only a minor part of the patients have a PET-CT examination. However, PET-CT plays a role in radiotherapy planning and in the detection of recurrence [Citation22,Citation23].
Another imaging modality is the dwMRI, which may play a future role in discriminating between benign and metastatic enlarged lymph nodes [Citation24,Citation25]. DwMRI is only recommended in three centres, but is performed at seven centres, suggesting an interest in adapting new techniques.
This study focused on the imaging of the neck. However, in the clinical work-up the imaging modality of choice may focus on visualising the primary site. The close anatomical relation to the teeth, mandible and maxilla complicates imaging of tumour in the oral cavity, inherently giving problems with dental artefacts, determination of bone involvement and neural invasion. Ideally, a single imaging modality would provide a complete evaluation of the extension of the primary tumour, detection of metastatic lymph nodes and distant metastases, as well as for irradiation planning [Citation26]. In cases where two or more imaging examinations are performed, also economical expenses must be considered and weighed against the clinical benefits.
This study has some limitations. First, the questionnaire may have been interpreted different ways in different centres. This is a well-known shortcoming of questionnaire-surveys, which we tried to address by asking the responders to specify which imaging modalities the guidelines recommend. Since the response rate to our questionnaire was 100% it is unlikely that responders refused to answer because of unclear questions. Second, the questionnaires were predominantly answered by ENT surgeons (19 of 21), since they first see the patients. Consequently, the surgeons may underestimate the percentage of PET-CT, since this imaging is most often performed after treatment decision and prior to radiotherapy.
In conclusion this study shows that CT is the most widely recommended and used imaging modality for oral squamous cell carcinomas but additional examinations are often performed. The availability of national guidelines does not ensure uniform performance of imaging, but well-defined guidelines including fast-track programme may improve the access to imaging. Moreover, a uniform work-up strategy and well-defined radiological criteria could provide future opportunities for pooling data in order to assess and improve the accuracy of the radiological work-up.
http://informahealthcare.com/abs/doi/10.3109/0284186X.2011.640346
Download PDF (39.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- NORDCAN [Internet]. Association of the Nordic Cancer Registries; 2009. Available from: http://www-dep iarc fr/NORDCAN/DK/frame.asp.
- DSHHO [Internet]. Behandling af orale planocellulære karcinomer. 2009. Available from: http://www dshho dk/files/OralRefProg_24juni2003 pdf
- Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+. Laryngoscope 2005;115:629–39.
- UICC. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
- Steinkamp HJ, Keske U, Schedel J, Hosten N, Felix R. [The spiral CT of cervical lymph node enlargements. The initial clinical results]. Rofo 1994;160:500–5. German.
- Available from: http://www.goscandinavia. http://goscandinavia about com/od/visatraveldocumentatio1/qt/nordicpopulation.htm. 2009.
- Kristiansen IS, Pedersen KM. [Health care systems in the Nordic countries – more similarities than differences?]. Tidsskr Nor Laegeforen 2000;120:2023–9. Norwegian.
- Bilde A, von BC, Johansen J, Bastholt L, Sorensen JA, Marker P, . The Danish national guidelines for treatment of oral squamous cell carcinoma. Acta Oncol 2006;45:294–9.
- Primdahl H, Nielsen AL, Larsen S, Andersen E, Ipsen M, Lajer C, . Changes from 1992 to 2002 in the pretreatment delay for patients with squamous cell carcinoma of larynx or pharynx: A Danish nationwide survey from DAHANCA. Acta Oncol 2006;45:156–61.
- Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol 2007;84:5–10.
- Kowalski LP, Carvalho AL. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol 2001;37:94–8.
- Sharp L, Lewin F, Hellborg H, Lundgren J, Hemmingsson E, Rutqvist LE. When does my treatment start? – The continuum of care for patients with head and neck cancer. Radiother Oncol 2002;63:293–7.
- Toustrup K, Lambertsen K, Birke-Sorensen H, Ulhoi B, Sorensen L, Grau C. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol Epub 2011 Jan 24.
- Sundhedsstyrelsen. Pakkeforløb for hoved-hals kræft. 2009. Available from: http://www sst dk/publ/Publ2009/SUPL/Pakke_kraeft/Kraeft_hovedhals_okt09 pdf.
- de Bondt RB, Nelemans PJ, Hofman PA, Casselman JW, Kremer B, van Engelshoven JM, . Detection of lymph node metastases in head and neck cancer: A meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol 2007;64:266–72.
- van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: How reliable is it? AJNR Am J Neuroradiol 1998;19:695–700.
- Yamazaki Y, Saitoh M, Notani K, Tei K, Totsuka Y, Takinami S, . Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med 2008;22:177–84.
- Zhu LJ, Chen ZW, Hou QY, Wang QP, Jiang S, Feng H, . [Clinical value of PET in identifying cervical nodal metastases of tongue cancer: a comparison with CT/MRI and clinical palpation in 38 cases]. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:2228–30. Chinese.
- Liao CT, Wang HM, Huang SF, Chen IH, Kang CJ, Lin CY, . PET and PET/CT of the neck lymph nodes improves risk prediction in patients with squamous cell carcinoma of the oral cavity. J Nucl Med 2011;52:180–7.
- Schroeder U, Dietlein M, Wittekindt C, Ortmann M, Stuetzer H, Vent J, . Is there a need for positron emission tomography imaging to stage the N0 neck in T1–T2 squamous cell carcinoma of the oral cavity or oropharynx? Ann Otol Rhinol Laryngol 2008;117:854–63.
- Seitz O, Chambron-Pinho N, Middendorp M, Sader R, Mack M, Vogl TJ, . 18F-fluorodeoxyglucose-PET/CT to evaluate tumor, nodal disease, and gross tumor volume of oropharyngeal and oral cavity cancer: Comparison with MR imaging and validation with surgical specimen. Neuroradiology 2009;51:677–86.
- Delouya G, Igidbashian L, Houle A, Belair M, Boucher L, Cohade C, . (18)F-FDG-PET imaging in radiotherapy tumor volume delineation in treatment of head and neck cancer. Radiother Oncol Epub 2011 Aug 30.
- Schoder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 2009;50(Suppl 1):74S–88.
- Hermans R. Diffusion-weighted MRI in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg 2010;18:72–8.
- Fukunari F, Okamura K, Zeze R, Kagawa T, Hashimoto K, Yuasa K. Cervical lymph nodes with or without metastases from oral squamous carcinoma: A correlation of MRI findings and histopathologic architecture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:890–9.
- Genden EM, Ferlito A, Silver CE, Takes RP, Suarez C, Owen RP, . Contemporary management of cancer of the oral cavity. Eur Arch Otorhinolaryngol 2010;267:1001–17.