Abstract
Background and purpose. To evaluate the patterns of locoregional relapse and survival following submandibular gland (SMG)-sparing intensity modulated radiotherapy (IMRT). Patients and methods. Eighty patients with laryngeal (n = 15), oropharyngeal (n = 50), hypopharyngeal (n = 11) or nasopharyngeal cancer (n = 4) were treated by submandibular gland-sparing IMRT for head and neck squamous cell cancer between July 2000 and December 2008. All patients were treated by bilateral IMRT. Thirty-nine (49%) received definitive radiotherapy (RT) and 41 (51%) postoperative RT. The contralateral parotid gland (PG) and SMG were included in the dose optimization planning program with intent to keep the mean doses for PG and SMG below 23 Gy and 28–30 Gy, respectively. The ipsilateral glands were also spared when considered feasible. Results. During a median follow-up time of 51 months (range, 24–117 months) nine local recurrent tumors were observed. Four of these nine patients were salvaged by surgery with no further recurrence. All local recurrences were located within the high-dose CTVs. None of the locally recurrent cancers were located at the vicinity of the spared PGs or SMGs. No recurrent tumors were observed in the contralateral neck. The Kaplan-Meier estimate for local control at five years following IMRT was 88% for the whole cohort and the corresponding figure for local control following salvage surgery was 94%. The estimates for five-year overall survival and disease-specific survival were 85% and 90%, respectively. Conclusion. In selected head and neck cancer patients who are estimated to have a low risk of cancer recurrence at the nodal levels I–II and who are treated with SMG-sparing IMRT the risk of cancer recurrence at the vicinity of the spared salivary glands is low.
During the last decade intensity modulated radiotherapy (IMRT) has become the standard radiotherapy (RT) technique in the treatment of head and neck cancer. IMRT enables the production of highly conformal dose distributions with less radiation- related late side effects to normal structures as compared to conventional radiation therapy at the vicinity of target volumes. A possibility to lower the cumulative radiation dose to the major salivary glands, resulting in a lower incidence of postirradiation xerostomia, is of particular interest.
Since the salivary glands are considered to function as parallel organs with respect to late radiation-induced effects [Citation1,Citation2], preservation of the salivary function can be expected if irradiation of large volumes of the major salivary glands can be avoided. The parotid glands (PG) produce most of the stimulated saliva, and their secretion is maximal when eating. Submandibular glands (SMG) produce up to 90% of the unstimulated salivary output. The composition of saliva secreted by the PGs and SMGs also differs. The PGs produce purely serous, watery saliva, whereas the saliva secreted by the SMGs contains mucins, which influence the degree of sensation of mouth dryness. Therefore, maintaining of the normal SMG function is essential to reduce postirradiation xerostomia.
Sparing of the SMGs is more challenging compared to sparing the PGs in RT planning. The SMGs are often located within or close to the target volumes of the primary tumor and the regional lymphatic tissues. The frequent proximity of the level II nodes, which are the most common site of nodal metastasis in head and neck squamous cell cancer (HNSCC), makes submandibular gland-sparing technically demanding. In our previous study on SMG-sparing using IMRT [Citation3] we concluded that SMG-sparing is feasible in selected patients and was not associated with locoregional recurrence within the spared volume. A D50 value for saliva secretion of 32.6 Gy at six months and 34.6 Gy at 12 months after completion of IMRT was estimated for the SMGs, which was somewhat higher than the D50 of the parotid glands (22.4 Gy and 27.4 Gy, respectively). The study was, however, based only on 18 patients who had a spared contralateral SMG using IMRT, and, therefore, were now evaluated the local control rate in a series of 80 patients treated with an identical protocol. To our knowledge, the current study reports the largest series of patients treated using SMG-sparing IMRT.
Material and methods
Patients
This study is based on a cohort of 80 patients with histologically diagnosed squamous cell carcinoma of the oropharynx (n = 50, 63%), larynx (n = 15, 18%), hypopharynx (n = 11, 14%) or nasopharynx (n = 4, 5%), who were treated using SMG-sparing IMRT at the Department of Oncology, Helsinki University Central Hospital, Finland, from July 2000 to December 2008. All patients with HNSCC treated with bilateral salivary gland-sparing IMRT within this time period were included in the study. The mean age of the patients at the time of the diagnosis was 59 years (range, 30–79). Twenty-two (28%) were female and 58 (73%) male. Thirty-nine (49%) patients were treated with definitive radiation therapy and 41 (51%) with postoperative RT. The main patient and tumor characteristics are presented in .
Table I. Patient and tumor characteristics.
The RT doses to the clinical target volumes (CTV) and to the parotid and submandibular glands were calculated from the dose-volume histograms (DVHs). The mean dose to the total volume of each PG and SMG was defined. The mean cumulative radiation dose delivered to the volume of each salivary gland correlates well with the residual function of the gland [Citation3,Citation4].
Pretreatment evaluation of the patients included clinical examination, head and neck imaging (MRI with or without computed tomography [CT]) and endoscopy. The tumors were staged according to the International Union Against Cancer (UICC) Tumour-Node Metastasis (TNM) classification [Citation5]. Treatment-related toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (http://ctep.cancer.gov).
The patients were regularly followed up in an outpatient department at three to six month intervals after RT; clinical examination was carried out at each visit. The median follow-up time was 51 months (range, 24–117 months). The sites of recurrent tumors were compared to the sites of the salivary glands and the clinical target volume of the radiation therapy treatment plan using MRI and CT images, and also positron emission tomography (PET)-CT images when available. All cancer recurrences were detected at radiological imaging, and were verified by a biopsy and histological examination of the tissue.
This study is a part of the IMRT quality assurance study approved by a Research Ethics Board of the Helsinki University Central Hospital.
Radiotherapy
For IMRT, the patients were fixed using a thermoplatic mask (Posicast®, Sinmed BV, EM Reenwijk, the Netherlands) until the year 2001, and thereafter a stereotactic head and neck immobilization device (BrainLab, Heimstetten, Germany) was used. The treatment isocenter was localized following computation of the stereotactic coordinates with the BrainScan® stereotactic treatment planning system (BrainLab). Treatment position was verified by two orthogonal simulator images taken weekly and, since 2005 by weekly portal imaging. Positional tolerance of 3 mm was chosen. Treatment planning CT was done using a slice thickness of 5.0 mm until the year 2005, and since 2005 a thickness of 2.5 mm. Irradiation was performed with a 6 MV linear accelerator using a dynamic multileaf collimator (dMLC) and the sliding window principle.
The target volume was delineated with the treatment planning CT images and diagnostic CT and/or MRI scans. When planning postoperative radiation therapy, the preoperative images were examined to determine the location of the primary tumor and the nodal metastases prior to surgery. In definitive IMRT the clinical target volume (CTV1) typically first encompassed the primary tumor with a 10 mm margin and the locogerional nodal sites. In postoperative IMRT planning a margin of 10 mm was added to the resection site to obtain the CTV1. A 3–5 mm margin was added to the CTV1 to obtain the planning target volume (PTV1). These target volumes were irradiated to a cumulative dose of 50 Gy, given in 2 Gy fractions over five weeks. The CTV2 and CTV3 encompassed the primary tumor and nodal metastases with a margin of 5–10 mm. In postoperative treatment the CTV2 and CTV3 encompassed the resection site of the primary tumor and the sites considered to be at a high-risk for nodal recurrence (e.g. sites with a nodal metastasis with extracapsular cancer infiltration at histopathological specimen assessment). The PTV2 and PTV3 were obtained from the CTV2 and CTV3 by adding a further margin of 3–5 mm. The PTV2 and PTV3 volumes were irradiated up to a total cumulative dose of 66–70 Gy. The maximum cumulative dose to the spinal cord allowed from IMRT was 40 Gy. All dose prescriptions were based on the International Commission on Radiation Units (ICRU) recommendations [Citation6].
The organs at risk (OAR) were defined in all slices. The contralateral PG and the SMGs were defined as OARs and were included in the dose optimization planning program with intent to keep the mean doses below 23 Gy and 28–30 Gy, respectively. The ipsilateral glands were also spared when feasible and provided that this did not lead to a PTV minimum dose that was less than 90% of the maximum dose, or a CTV dose less than 95% of the maximum.
Concomitant chemotherapy
Thirty-eight (97%) of the 39 patients treated with definitive IMRT and 21 (51%) of the 41 patients treated with postoperative IMRT received concomitant chemotherapy. Patients with nasopharyngeal cancer treated with definitive chemoradiotherapy (n = 4) received intravenous cisplatin 100 mg/m2 administered three-weekly during IMRT. The rest of the patients were scheduled to receive weekly intravenous cisplatin 40 mg/m2 six times during the RT course. The indications for concomitant chemotherapy in the postoperative setting were marginal resection, presence of more than one metastatic lymph node in the surgical specimen, and extracapsular growth of nodal metastasis. Prophylactic antiemetics were prescribed for all patients at the time of chemotherapy.
Statistical analyses
The SPSS statistical program (version 17.0, Chicago, IL, USA) was used for statistical calculations. One-way ANOVA was used to compare the RT doses received by the salivary glands. Survival was estimated using the Kaplan-Meier product-limit method. Cox regression analysis was used to calculate the significance of patient and tumor characteristics on local control and survival. Local recurrence-free survival as calculated from the date of RT completion to the date of first locoregional recurrence or death, censoring patients alive without local recurrence on the last date of follow-up. Local control was defined as absence of primary tumor and regional nodal metastasis at physical examination, endoscopy and imaging. Overall survival was calculated from the last date of RT to the date of death. Disease-free survival was calculated from the last date of RT to death caused by head and neck cancer, censoring patients who were alive on the date of last follow-up and those who died from another cause on the date of death.
Results
Cumulative target volume and salivary gland doses
The mean total cumulative target dose delivered with definitive IMRT was 69.5 Gy (range, 66–70 Gy) and the mean overall treatment time was 50 days (range, 45–60 days). The mean cumulative dose delivered to the PTV1 was 50 Gy (n = 39), to the PTV2 10–20 Gy (n = 39) and to the PTV3 4–6 Gy (n = 13). In the subset of patients who received IMRT postoperatively (n = 41), the mean cumulative dose was 58 Gy (range, 50–70 Gy) and the mean overall treatment time 43 days (range, 30–56 days). In this subset, the mean cumulative dose delivered to the PTV1 was 50 Gy (n = 41), to the PTV2 8 Gy (range, 4–16 Gy; n = 40), and to the PTV3 5 Gy (range, 4–6 Gy; n = 4).
In 37 (90%) of the 41 patients treated with postoperative RT the ipsilateral SMGs were removed at neck dissection, whereas the rest of the patients (n = 43) had intact PGs and SMGs. The numbers of salivary glands spared at IMRT by the primary tumor site are provided in and the mean doses received by these glands in . Patients with larynx cancer received lower cumulative doses to the ipsilateral and contralateral PGs and to the ipsilateral SMGs compared to the rest of the patients (p = 0.001), whereas the cumulative doses to the spared contralateral SMGs were similar (p = 0.62, ).
Figure 1. The mean cumulative total radiation doses to the contralateral parotid gland (cPG), contralateral submandibular gland (cSMG), ipsilateral parotid gland (iPG) and ipsilateral submandibular gland (iSMG).
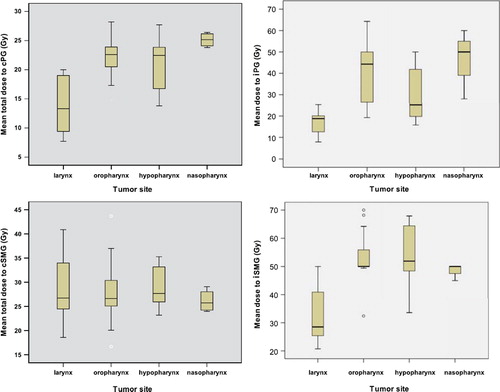
Table II. The numbers of spared parotid and submandibular glands by the primary tumor site.
Table III. Mean doses to the spared salivary glands by the primary tumor site.
Local control and patterns of relapse
Cancer recurred locally in six (15%) of the 39 patients treated with definitive IMRT and in three (7%) of the 41 patients who received postoperative IMRT. Two patients treated for T3N0 glottic laryngeal cancer by curative intent chemoradiotherapy up to a total dose of 70 Gy to the primary tumor site had relapse at the site of the original tumor and underwent total laryngectomy as salvage surgery. The recurrences were classified as rT2 and rT4. In both cases the site of recurrence was situated at the CTV70 volume of RT. Both patients were disease-free at 37 and 42 months following laryngectomy. Two patients with T1N2b and T4N0 hypopharyngeal cancer also had locoregional recurrence. The first of these patients recurred at ipsilateral neck at the level IV within the CTV60 volume; this patient had concomitant distant metastases (bone and soft tissue). The ultimate cause of death was cancer (local recurrence and distant metastases). The second patient had residual tumor following RT at the original tumor site within the CTV70 volume and this patient died of local recurrence. Two patients with definitive IMRT for T2N2a and T2N2b cancer of the base on tongue also had recurrence. In the first patient residual tumor was present ipsilaterally at the original nodal level II disease site within the CTV70 volume and was treated by radical neck dissection. The patient is alive without evidence of disease at 38 months following salvage surgery. The second patient had ipsilateral nodal recurrence within the CTV60 volume together with lung metastasis.
Locoregional tumor recurrence was observed also in three patients treated by postoperative IMRT. Two of these had tonsillar cancer, T3N1 and T4N2b, respectively. The first patient had local recurrence at the site of the primary tumor within the CTV60 volume and the CTV50 nodal volume together with lung metastases. The patient with T4N2b cancer had recurrence at the primary tumor site within the CTV60 nodal volume. This patient was treated by salvage surgery and has no evidence of disease at 26 months following surgery. The third patient was operated for T2N1 cancer of the soft palate and developed ipsilateral nodal recurrence within the CTV50 nodal treatment volume. The patient died of local recurrence.
None of locally recurrent cancers were located at the vicinity of the spared PGs or SMGs. No recurrent tumors were detected in the contralateral neck. In a Cox regression model the mean IMRT doses delivered to the PGs and SMGs were not associated with the risk of local recurrence (p = 0.45, 0.97, 0.98 and 0.85 for the ipsilateral PGs, the contralateral PGs, the ipsilateral SMGs and the contralateral SMGs, respectively). Of the tumor-related factors, tumor size (T-classification) was significantly associated with local recurrence (p = 0.042) and cancer stage (stage I–II vs. III–IV) tended to be significant (p = 0.063), whereas nodal stage was not (p = 0.26). Neither patient age nor sex was significantly associated with locoregional recurrence (p = 0.79 and 0.66, respectively).
Survival analyses
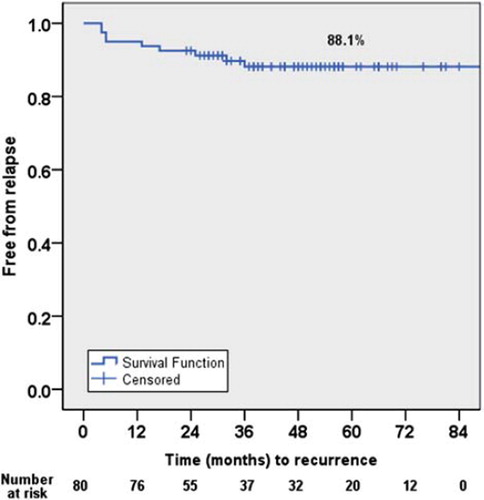
Fourteen (18%) of the 80 patients died during the follow-up. In seven of these 14 patients the cause of death was recurrent cancer. The cause of death was cancer local recurrence in two cases, local recurrence together with distant metastasis in three, and distant metastases without local tumor recurrence in two patients. Three patients died from a second primary cancer, one from pneumonia, one from rhabdomyolysis and two from a cardiovascular disease. The Kaplan-Meier estimate for five-year overall survival was 85% and for five-year disease-specific survival 90%. The Kaplan-Meier estimate for local control five years following IMRT was 88% for the whole cohort (), and the corresponding figure for local control following IMRT plus salvage surgery was 94%.
Acute and late toxicity
The most frequent radiation-related acute side effects were skin and mucosal irritation. Mucositis was graded as Grade 1 or 2 in 60 (75%) of the 80 patients and Grade 3 in 25%. Dermatogical toxicity was mild, and no toxicity exceeding Grade 2 was observed.
Fifty-nine (74%) of the 80 patients received concomitant chemotherapy, all of whom received cisplatin. The most frequent chemotherapy-related acute adverse events were nausea, and bone marrow and renal toxicity. Grade 1 to 2 nausea occurred in 38 (64%) of the 59 patients and Grade 3 in four (7%). Mild to moderate (Grade 1 to 2) increase in serum creatinine occurred in 21 (36%) of the 59 patients. None of the patients developed permanent renal insufficiency. Grade 3 to 4 neutropenia was observed in seven (12%) patients, mild to moderate thrombocytopenia in 42 (71%) and Grade 1 to 2 anemia in 52 (88%). Reversible mild ototoxicity was reported in two (3%) patients and neurotoxicity in three (5%).
No cases of osteoradioradionecrosis were reported during the follow-up period. The quality of voice in patients treated for laryngeal cancer who survived with preserved larynx (13 of the 15 patients with larynx cancer) was estimated to be good in seven patients, slightly or moderately hoarse in five and poor in one patient. Permanent xerostomia higher than grade 2 was not reported in any of the patients. Five patients had problems in swallowing with inability to eat solid food. However, only one patient treated by postoperative IMRT for T3N2b tonsillar cancer needed a permanent percutaneus endoscopic gastrostomy (PEG).
Discussion
After the introduction of IMRT to the treatment of head and neck cancer, preservation of the parotid gland function by sparing at least the contralateral parotid gland from high doses of irradiation has been under intensive study [Citation2,Citation5,Citation7,Citation8]. However, the data on submandibular gland-sparing with IMRT are scant, and no large studies have been published thus far. In the present series 80 patients treated by submandibular gland-sparing IMRT were analyzed for local recurrence rate, survival, and adverse events related to IMRT. We found a low risk for local cancer recurrence at levels I–II, and, importantly, no recurrences in the vicinity of the spared salivary gland or in the contralateral neck.
In a prospective study Jaguar et al. evaluated the impact of SMG resection on the salivary gland function. The unstimulated salivary flow decreased significantly following surgery whereas the stimulated salivary flow did not [Citation9]. The results of surgical transfer of SMG into the submental space prior to RT for head and neck cancer were published by Jha et al. [Citation10]. Their conclusion was that surgical transfer of a SMG into the submental space preserves function and prevents the development of radiation-induced xerostomia. In a further study where gland transfer was combined with helical tomotherapy, the mean dose to the total parotid volume and the transferred SMG could be reduced to less than 26 Gy [Citation11]. The potential advantage of this approach is that the selective neck dissection performed at the levels I–III on the side chosen for gland transfer prior to RT rules out microscopic cancer in the vicinity of the spared SMG.
Attempts have been made to reduce the risk of postirradiation xerostomia by minimizing the RT doses to the contralateral SMG or, in selected cases, to both SMGs with new RT techniques. In our previous study on 18 patients with SMG-sparing IMRT, no recurrences were observed in the vicinity of the spared salivary glands [Citation3]. In a planning study Houweling et al. concluded that reducing the mean cumulative dose to the SMG below 40 Gy is possible using IMRT while maintaining reasonable dose coverage to the elective contralateral PTV [Citation12]. Jellema et al. studied the association between the mean radiation dose to the salivary glands, patient-rated xerostomia and stickiness of saliva, and observed that the occurrence of sticky saliva depended mainly on the mean dose to the SMGs [Citation13]. Deasy et al. reviewed publications on RT dose-volume effects on the salivary gland function. Their recommendation was that the mean dose to each parotid gland should be kept as low as possible and consistent with the desired CTV coverage, and when deemed safe, submandibular gland-sparing using modest cumulative doses (< 35 Gy) might reduce xerostomia symptoms [Citation14]. A recent prospective study on SMG-sparing IMRT by Wang et al. concluded that the recovery of the saliva output was better and the grade of postIMRT xerostomia lower in patients whose contralateral SMGs were spared as compared to those whose glands were not spared [Citation15]. Twenty-six of the 52 patients in this study were treated by SMG-sparing IMRT, most (n = 47) received postoperative IMRT and five definitive IMRT. The influence of the mean doses to the cSMG and cPG on the recovery of saliva output was equivalent to that of the mean volume of the gland receiving 30 Gy. No recurrences in the vicinity of the spared PGs and SMGs were observed. The dose-effect relationships and the implications for SMG-sparing were addressed in yet another recent study, which concluded that the SMG salivary flow rates depended on the mean cumulative gland dose and that the saliva flow recovers with time provided that the cumulative gland dose is kept under a threshold dose of 39 Gy [Citation16]. Substantial reduction of the dose to levels below this threshold was considered feasible in some patients without target underdosing, but at the expense of modestly higher doses to some other organs. In the present study the mean cumulative doses to the spared salivary gland volumes could be kept at or below the threshold levels suggested by prior studies [Citation3,Citation4,Citation12–16].
We found no cancer recurrences near the spared SMG and PG glands. All nodal recurrences occurred at the site of the original nodal disease within the high-dose CTVs. The same was found for the primary tumor relapses, all of which occurred at the site of the original tumor or, following postoperative RT, in the surgical bed of the primary tumor irradiated to a high dose. More importantly, none of the nodal recurrences occurred in the contralateral neck. In the treatment of glottic cancer, sparing of both PGs and SMGs seems feasible in most cases, since the frequency of nodal metastasis is low and the location of the primary tumor is caudal to the spared SMGs. With the exception of the contralateral SMG, the mean doses to the spared salivary glands with were lower in patients with laryngeal cancer as compared to patients who had a tumor at another site. In hypopharyngeal cancer both SMGs can occasionally be spared when the tumor is small, but since such patients have frequently nodal metastasis at the level II, sparing should be done with caution. We attempted sparing of the ipsilateral SMG in only one of the 11 patients with hypopharyngeal cancer. In oropharyngeal HNSCC the most frequent site of nodal metastasis is the level II nodes which, together with the close proximity of the primary tumor, makes sparing of the ipsilateral SMG hazardous and can thus be considered only seldom.
We found no association between the mean doses delivered to the salivary glands and the local control. This was probably due to careful selection of patients scheduled to receive SMG-sparing IMRT. Sparing was not attempted in patients who were considered to have a high risk of recurrence at contralateral level II lymph nodes in the vicinity of the cSMG or, when ipsilateral SMG-sparing was considered, in the ipsilateral level II nodes. We were also cautious with tumors with a risk of involvement of level I nodes, such as cancers of the oral tongue or the floor of the mouth.
Accurate delineation of the salivary glands is essential in salivary gland-sparing IMRT. Houweling et al. demonstrated that the delineation of the submandibular glands was improved in the cranial direction by using T1- and T2-weighted MRI and MRI sialography [Citation17]. Besides the accurate gland delineation methods, correct patient set-up is important to achieve optimal results [Citation18–20]. When a substantial part of a major salivary gland function is planned to be spared, the dosimetric and positional tolerance levels should be stringent due to the steep dose response curve in the glands [Citation20]. Integration of combined functional and anatomical imaging modalities, such as PET-CT and functional MRI, into the RT treatment planning likely improves treatment accuracy and facilitates evaluation of tumor and normal tissue responses to RT [Citation21–24].
Conclusion
We conclude that in selected head and neck cancer patients who are estimated to have a low risk of cancer recurrence at the nodal levels I–II and who are treated with SMG-sparing IMRT, the risk of cancer recurrence at the vicinity of the spared salivary glands is small. In the current study, consisting of 80 such patients, we did not detect any recurrences.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys 1998; 14:751–9.
- Yorke ED, Kutcher GJ, Jackson A, Ling CC. Probability of radiation-induced complications in normal tissues with parallel architecture under conditions of uniform whole or partial organ irradiation. Radiother Oncol 1993;26:226–37.
- Saarilahti K, Kouri M, Collan J, Kangasmaki A, Atula T, Joensuu H, . Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol 2006;78:270–5.
- Saarilahti K, Kouri M, Collan J, Hämäläinen T, Atula T, Joensuu H, . Intensity modulated radiotherapy for head and neck cancer: Evidence for preserved salivary gland function. Radiother Oncol 2005;74:251–8.
- Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th ed. UICC, Wiley-LISS: New York; 2002.
- ICRU. International Commission on Radiation Units and Measurements Report 50: Prescribing, recording and reporting photon beam therapy. Washington, DC: ICRU; 1993.
- Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys 2001; 51:938–46.
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87.
- Jaguar GC, Lima ENP, Kowalski AC, Pellizon AC, Carvalho AL, Alves FA. Impact of submandibular gland excision on salivary gland function in head and neck cancer patients. Oral Oncol 2010;46:349–54.
- Jha N, Seikaly H, Harris J, Williams D, Liu R, McGaw T, . Prevention of radiation induced xerostomia by surgical transfer of submandibular gland into the submental space. Radiother Oncol 2003;66:283–9.
- Saibishkumar E, Jha N, Scrimger R, MacKenzie M, Daly H, Field C, . Sparing the parotid glands and surgically transferred submandibular gland with helical tomotherapy in post-operative radiation of head and neck cancer: A planning study. Radiother Oncol 2007;85:98–104.
- Houweling A, Dijkema T, Roesink J, Terhaard C, Raaijmakers C. Sparing the contralateral submandibular gland in oropharyngeal cancer: A planning study. Radiother Oncol 2008; 89:64–70.
- Jellema A, Doornaert P, Slotman B, Leemans C, Langendijk J. Does radiation dose to the salivary glands and oral cavity predict patients-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol 2005;77:164–71.
- Deasy J, Moiseenko V, Marks L, Chao K, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010;76:S58–63.
- Wang ZH, Yan C, Zhang ZY, Chang CP, Hu HS, Tu WY, . Impact of salivary gland dosimetry on post-IMRT recovery of saliva output and xerostomia grade for head-and-neck cancer patients treated with or without contralateral submandibular gland sparing: A longitudinal study. Int J Radiat Oncol Biol Phys Epub 2010 Oct 7.
- Murdoch-Kinch C-A, Kim H, Vineberg K, Ship J, Eisbruch A. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72:373–82.
- Houweling A, van der Berg C, Roesink J, Terhaard C, Raaijmakers C. Magnetic resonance imaging at 3.0 T for submandibular gland sparing radiotherapy. Radiother Oncol 2010;97:239–43.
- Johansen J, Bertelsen A, Hansen C, Westberg J, Hansen O, Brink C. Set-up errors in patients undergoing image guided radiation treatment. Relationship to body mass index and weight loss. Acta Oncol 2008;47:1454–8.
- Mongioj V, Orlandi E, Palazzi M, Deponti E, Marzia F, Stucchi C, . Set-up errors in IMRT treatments for nasopharyngeal carcinoma to evaluate time trends, PTV and PRV margins. Acta Oncol 2011;50:61–71.
- Kapanen M, Collan J, Saarilahti K, Heikkonen J, Kairemo K, Tenhunen M. Accuracy requirements for head and neck intensity-modulated radiation therapy based on observed dose response of the major salivary glands. Radiother Oncol 2009;93:109–14.
- Eilertsen K, Vestad L, Geier O, Skretting A. A simulation of MRI based dose calculations on the basis of radiotherapy planning CT images. Acta Oncol 2008;47:1294–302.
- Partridge M, Yamamoto T, Grau C, Høyer M, Muren L. Imaging of normal lung, liver and parotid gland function for radiotherapy. Acta Oncol 2010;49:997–1011.
- Newbold K, Partridge M, Cook C, Sharma B, Rhys-Evans P, Karrington K, . Evaluation of 18FDG-PET in radiotherapy target definition in patients with head and neck cancer. Acta Oncol 2008;47:1229–36.
- Røe K, Aleksandersen T, Kristian A, Nilsen L, Seierstad T, Qu H, . Preclinical dynamic 18FDG-PET – tumor characterization and radiotherapy response assessment by kinetic compartment analysis. Acta Oncol 2010;49:914–21.