Abstract
Background. The survival advantage achieved by existing anti-cancer agents as second-line therapy for relapsed non-small cell lung cancer (NSCLC) is modest and further improvement of treatment outcome is desired. Combination chemotherapy with irinotecan and amrubicin for advanced NSCLC has not been fully evaluated. Methods. The primary endpoint of this phase II clinical trial was objective response. Patients with NSCLC who had been treated previously with one or two chemotherapy agents were enrolled. Irinotecan and amrubicin were both administered on Days 1 and 8 of a 21-day cycle, at doses of 100 mg/m2 and 40 mg/m2, respectively. Results. Between 2004 and 2006, 31 patients received a total of 101 courses; the median number of courses administered was three (range, one to six). Objective response was obtained in nine of the 31 patients (29.0% response rate; 95% confidence interval (CI), 12.1–46.0%). With a median follow-up time of 43.9 months, median survival time and the median progression-free survival time were 14.2 and 4.0 months, respectively. Myelosuppression was the most frequently observed adverse event, with grade 3/4 neutropenia in 51% of patients. Febrile neutropenia developed after nine courses (9%) and resulted in one treatment-related death. Cardiac toxicity and diarrhea, possibly specific for both agents, were infrequent and manageable. Conclusion. Combination chemotherapy with irinotecan and amrubicin is effective in patients with NSCLC but showed moderate toxicities in second- or third-line settings.
The standard treatment for relapsed non-small cell lung cancer (NSCLC) has historically been docetaxel monotherapy [Citation1]. Thereafter, treatment with pemetrexed and the epidermal growth factor receptors-tyrosine kinase inhibitors (EGFR-TKI) gefitinib and erlotinib has shown efficacy similar to that of docetaxel in several phase III clinical trials [Citation2–4]. However, the increase in survival achieved by these chemotherapy regimens was very modest and further improvement in treatment outcome is desired. Additionally, several agents with unique mechanisms are now available and have been shown to be highly effective in treating NSCLC [Citation5]. Irinotecan is a semi-synthetic, water-soluble derivative of camptothecin, which inhibits topoisomerase I, an enzyme that relaxes DNA by inducing single-strand DNA breaks [Citation6]. This compound is also active for NSCLC, both as a single agent and in combination with cisplatin (CDDP) [Citation7–9]. Amrubicin, a totally synthetic anthracycline derivative characterized by a 9-amino group and a simple sugar moiety, is converted in the body to amrubicinol by reduction of the 13-position ketone, leading to higher anti-tumor activity (25% response rate) [Citation10]. Although classified as anthracycline agents, amrubicin and amrubicinol exert cytotoxic effects as DNA topoisomerase II inhibitors, not only as DNA intercalators. The combined use of topoisomerase I and II inhibitors has been demonstrated to be complementary in preclinical studies [Citation11,Citation12], and in vitro simultaneous administration of both agents has an advantage for cytokilling [Citation13].
On the basis of these results, we conducted a phase I clinical trial assessing the combination of irinotecan and amrubicin in patients with advanced NSCLC [Citation14]. This regimen was well tolerated, and the recommended doses for phase II studies were 40 mg/m2 amrubicin, followed by 100 mg/m2 irinotecan, given on Days 1 and 8 of a 21-day cycle. In the phase I clinical trial, an objective response was observed in the relapsed patients but not in the chemo-naïve patients, but no definitive reason for this difference was found.
Here, we report the results of a phase II clinical trial using this combination chemotherapy in patients with relapsed NSCLC. The primary objective was to determine the response rate for this combination. Secondary objectives included overall survival time (OS), progression-free survival (PFS) times, and safety in the second- or third-line setting.
Patients and methods
Eligibility criteria
To be enrolled in the trial, patients had to meet the following eligibility criteria: pathologically proven, advanced, and inoperable NSCLC; measurable lesions; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; age ≤ 75 years; adequate reserves of hematologic function [white blood cell (WBC) count > 4000/μl, neutrophil count > 2000/μl, hemoglobin level > 9.5 g/dl, platelet count > 10 × 104/μl], renal function (serum creatinine < 1.5 mg/dl), hepatic function (total bilirubin < 1.5 mg/dl, serum transaminases < 2.5 × upper limit of normal range) and pulmonary function (PaO2 ≥ 60 Torr); and provision of written informed consent. Patients who had undergone one or two previous chemotherapy regimens, but had no prior use of irinotecan and/or amrubicin, were eligible. Patients with symptomatic brain metastasis were excluded from the study. Baseline pretreatment evaluations included a complete history, physical examination, laboratory tests, chest radiograph, electrocardiogram, computed tomography (CT)-scans of the chest and abdomen, magnetic resonance imaging (MRI) of the brain, and a radionuclide bone scan. Positron emission tomography (PET)/CT was also used for staging in some cases. Staging was assessed according to the tumor, node, and metastasis [Citation15]. The protocol was approved by the institutional review board of each participating institute and performed in accordance with the Declaration of Helsinki (1964, amended in 2000) of the World Medical Association.
Treatment schedules
Based on the phase I study results [Citation14], 40 mg/m2 of amrubicin diluted in 20 ml of physiological saline was initially administered intravenously over a 5-min period on Days 1 and 8. Soon thereafter, 100 mg/m2 of irinotecan diluted in 250 ml of physiological saline was administered intravenously over a 1-h period on the same day. Treatment was repeated every 3 weeks. Each patient was premedicated with an intravenous administration of dexamethasone (8 mg) and granisetron (3 mg) 30 min before the bolus infusion of amrubicin. Treatment was repeated basically for four cycles.
The administration of irinotecan and amrubicin on Day 8 was delayed until Day 15 if hematological toxicity > grade 3, non-hematological toxicity > grade 2, or diarrhea was observed on the day of administration. If these toxicities did not improve by Day 15, therapy administration was cancelled during that course. The initiation of the next course of chemotherapy was delayed until the recovery of WBC count to > 3000/μl, neutrophil count to > 1500/μl, platelet count to > 10 × 104/μl, and the resolution of non-hematological toxicities to < grade 1. The use of granulocyte colony- stimulating factor (G-CSF) was permitted when grade 4 leukopenia, grade 4 neutropenia, or febrile neutropenia was noted.
Assessment of efficacy, toxicity, and anti-tumor activity
The Response Evaluation Criteria in Solid Tumors (RECIST; ver. 1.0) guideline was applied to evaluate responses. The best overall response was defined as the best response recorded from the start of treatment until disease progression or recurrence. Complete and partial responses were confirmed by two observations not less than 4 weeks apart. A determination of stable disease required disease stabilization for at least 6 weeks. All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (ver. 3.0).
Statistical considerations
Assuming that a response rate of 20% in eligible patients would indicate potential usefulness, whereas a rate of 5% would be the lower limit of interest, with α = 0.05 and ß = 0.20, the estimated accrual number was 27 patients. This regimen would be rejected when only one of the first 13 patients had an objective response at the interim analysis. With an assumed dropout rate of 10%, 30 patients were required. OS was defined as the interval between the date of enrollment in this study and the date of death or the last follow-up visit. PFS was defined as the interval between the date of enrollment and the date of the first observation of disease progression or death from any cause. The survival distribution was estimated using the Kaplan-Meier method [Citation16].
Results
Patient characteristics
A total of 31 patients with recurrent NSCLC were enrolled between November 2004 and June 2006 at six institutes. The baseline patient characteristics are shown in . Most patients were male and non-smokers, with a good PS and adenocarcinoma histological findings. The most dominant prior chemotherapy regimens were cisplatin-based chemotherapy as a first-line treatment and EGFR-TKI as a second-line therapy. The maximal responses to the prior chemotherapies were as followed: complete response 1, partial response 13, stable disease 3 and inevaluable 1, and the corresponding median PFS was 2.2 months. All patients and courses were assessable for safety and efficacy.
Table I. Patient characteristics.
Treatment delivery
In total, 101 treatment courses were administered. The median number of treatment courses was three, with a range of one to six courses. Dose and schedule modifications were necessary in 27 (27%) courses; dose reduction was required in 23 courses, administration was delayed in two courses, and chemotherapy was skipped on Day 8 in two courses.
Objective tumor response and overall survival
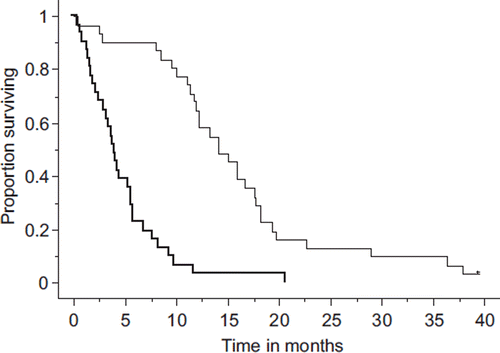
Tumor response was observed in nine patients, leading to an objective response rate of 29.0% [95% confidence interval (CI) 12.1–46.0%]. Among these nine patients, three were treated in the second-line setting (three of 13 patients, response rate: 23.1%) while the remaining six were treated in the third-line setting (six of 18 patients, response rate: 33.3%). Eighteen (58.1%) patients achieved disease stability, whereas nine (9.7%) developed disease progression. The OS periods of all treated patients are shown in . At the time of survival analysis, progression event was observed in all 31 patients while 28 of the 31 patients had died. The median follow-up time for surviving patients (censored patients) was 28.6 months (range, 27.7–33.9 months), the median survival time of the 31 treated patients was 14.2 months, and the 1-year survival rate was 64.5%. The median PFS time and 6-month PFS rate were 4.0 months and 22.6%, respectively ().
Figure 1. Survival curves: Bold and thin lines indicate progression-free and overall survival, respectively. The median survival time and 1-year survival rate were 14.2 months and 64.5%, respectively, while the median progression-free survival time and 6-month progression-free survival rate were 4.0 months and 22.6%, respectively.
Adverse events
Neutropenia was the principal hematological toxicity observed; half of all patients developed grade 3 or 4 neutropenia (). G-CSF was administered in 17 (17%) of the 101 courses. Grade 3 or 4 anemia and thrombocytopenia were less common, and transfusions to address these conditions were required in only two courses each.
Table II. Adverse events in the 31 patients with 101 courses.
The non-hematological toxicities were moderate: grade 3 or 4 febrile neutropenia occurred in seven patients (23%) with nine (9%) courses. Diarrhea was almost manageable, manifesting as grades 3–4 in four patients with four cycles; however, one patient who developed fatal diarrhea with febrile neutropenia died. Cardiac ischemia (vasospastic angina) developed in one patient with one course, but was reversible. Severe hepatic and pulmonary toxicities were rarely observed, and all of these toxicities were reversible with appropriate supportive care.
Discussion
Here, we have demonstrated that the combination of irinotecan and amrubicin using a fractionated administration schedule produced therapeutically relevant effects as a second- or third-line treatment option (29.0% response rate; 95% CI 12.1–46.0%). Additionally, this regimen was moderately tolerated; the principal toxicity observed was myelosuppression. Diarrhea and cardiac toxicity were also observed during several treatment courses, and one patient died.
The combination of irinotecan and amrubicin produced a high response rate and favorable survival in our study. The existing efficacy data for the standard regimen revealed a lower (< 10%) objective response rate and a median PFS time of < 3 months [Citation1–4]. The high response rate might be partly attributable to possible compensatory roles of both topoisomerase I and II enzymes: development of cellular resistance to topoisomerase II inhibitors conferred an increased sensitivity to topoisomerase I inhibitors [Citation11]. The reverse effect, in which resistance to a topoisomerase I inhibitor enhances the sensitivity to topoisomerase II inhibitors, has also been reported [Citation12]. Indeed, when administered simultaneously, some reports have indicated synergistic or additive cytotoxic effects in various tumor cell lines [Citation13]. A potential role by each topoisomerase enzyme in overcoming a shortage in the other would enhance sensitivity.
Another hypothesis for the favorable efficacy data is that neither irinotecan nor amrubicin was cross-resistant to the prior chemotherapy regimens, especially those using platinum, a key chemotherapeutic drug [Citation17–19] that had frequently been used in the first-line setting in our cohort (). In an in vitro study, both agents remained sensitive to cancer cells that were resistant to platinum [Citation20,Citation21]. Therefore, their non-cross-resistant activity led to favorable OS results. Additionally, assuming the discordant time interval between OS and PFS, it should be taken into consideration that the effects of post-study treatments and/or patient selection may have favorably affected patient survival. However, we have no available data regarding post-study treatments, which is one of the major limitations in this study.
Amrubicin is commonly administered intravenously for three consecutive days [Citation22,Citation23]. However, this approved dosing schedule is too toxic in relapsed patients; in a previous study, the use of amrubicin as a single agent led to febrile neutropenia in 35% of patients and one case of treatment-related pneumonia [Citation24]. For this reason, we considered the fractionated schedule of the two drugs to be less toxic and more manageable, and conducted the initial phase I clinical trial using separate administration [Citation14]. Another group also investigated the same combination but used a consecutive three-day administration of amrubicin [Citation25]. This led to unexpected severe toxicities, including myelosuppression, followed by infection, diarrhea, and pneumonitis. The doses of both agents could not be increased, and the maximum tolerated dose and the recommended dose could not be determined. The difference between these results and ours might be partly due to the difference in the treatment schedule, although other known and unknown confounding issues, including differences in patient selection and supportive care, may also have contributed.
As observed in our phase I clinical trial, myelosuppression was the major toxicity. However, the toxicity profiles of this regimen seemed moderate due to the relatively high proportion of febrile neutropenia and the single toxic death. Genetic variants in the uridine 5’-diphospho-glucuronosyltransferase 1A1 (UGT1A1) gene were associated with the risk of severe neutropenia caused by irinotecan [Citation26], and the UGT1A1*6 or *28 allele was observed in 13–17% of the Japanese population [Citation27]. This patient might have possessed the homozygous genotypes for UGT1A1*6 or *28, although we did not investigate the UGT1A1 gene status. Therefore, UGT1A1 gene status may need to be assessed during patient selection, prior to the administration of irinotecan, although such assessment is not currently required by the Japanese government prior to the administration of irinotecan.
One of the major toxicities associated with anthracyclines is cardiotoxicity [Citation28]. However, in a preclinical study using dogs, amrubicin had neither cardiotoxicity nor deteriorating effects on pre-existing cardiomyopathy [Citation29]. Also, previous clinical trials involving 74 patients with small cell lung cancer demonstrated that amrubicin had no cardiotoxicity [Citation30,Citation31]. In our trial, reversible vasospastic angina was observed in one patient and may have been treatment-related. Further assessment of this issue is required in a larger cohort.
In conclusion, a fractionated administration of irinotecan and amrubicin seemed highly effective for advanced NSCLC that had relapsed after first- or second-line treatment with platinum-based regimens, but was associated with moderate toxicities and one treatment-related death occurred. It might be useful in selected relapsed patients, especially who have already received docetaxel or pemetrexed, standard salvage chemotherapy regimen, in the earlier line setting, and who have adequate reserves of hematologic function. The development of appropriate tools for the selection of patients who would benefit most from this treatment regimen, and further improvement of the regimen's efficacy and toxicity profiles, are warranted.
Acknowledgements
The UMIN trial registration number is C000000131. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: Dr. Hotta was paid an honorarium for lecturing from Sanofi-Aventis Japan, Nihon Kayaku, Lilly Japan and Chugai pharmaceutical. Drs. Takigawa and Kiura were paid an honorarium for lecturing from Sanofi-Aventis and Chugai Pharmaceutical, Japan. The other authors report no conflict of interest.
References
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, . Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095–103.
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, . Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, . National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32.
- Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, . Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet 2008;372:1809–18.
- Bunn PA. Chemotherapy for advanced non-small-cell lung cancer: Who, what, when, why? J Clin Oncol 2002; 20:23s–33s.
- Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 1988;48:1722–6.
- Fukuoka M, Niitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, . Phase II study of irinotecan, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer. J Clin Oncol 1992;10:16–20.
- Negoro S, Masuda N, Takada Y, Sugiura T, Kudoh S, Katakami N, . Randomised phase III trial of irinotecan combined with cisplatin for advanced non-small-cell lung cancer. Br J Cancer 2003;88:335–41.
- Ueoka H, Tanimoto M, Kiura K, Tabata M, Takigawa N, Segawa Y, . Fractionated administration of irinotecan and cisplatin for treatment of non-small-cell lung cancer: A phase II study of Okayama Lung Cancer Study Group. Br J Cancer 2001;85:9–13.
- Negoro S, Fukuoka M, Nakamura S, Ikegami H, Sugiura T, Ariyoshi Y, . Phase I–II study of amrubicin (SM-5887), a novel 9-amino-anthracycline, by iv administration for 3 consecutive days in patients with advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 1995;14:abstr 1105.
- Tan KB, Mattern MR, Eng WK, McCabe FL, Johnson RK. Nonproductive rearrangement of DNA topoisomerase I and II genes: Correlation with resistance to topoisomerase inhibitors. J Natl Cancer Inst 1989;81:1732–5.
- Sugimoto Y, Tsukahara S, Oh-hara T, Liu LF, Tsuruo T. Elevated expression of DNA topoisomerase II in camptothecin-resistant human tumor cell lines. Cancer Res 1990;50: 7962–5.
- Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M, . Effects of CPT-11 in combination with other anti- cancer agents in culture. Int J Cancer 1992;50:604–10.
- Hotta K, Takigawa N, Kiura K, Tabata M, Umemura S, Ogino A, . Phase I study of irinotecan and amrubicin in patients with advanced non-small-cell lung cancer. Anticancer Res 2005;25:2429–34.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718–23.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non- small-cell lung cancer. J Clin Oncol 2004;22:3852–9.
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Addition of platinum compounds to a new agent in patients with advanced non-small-cell lung cancer: A literature based meta-analysis of randomised trials. Ann Oncol 2004;15:1782–9.
- Hotta K, Matsuo K. Long-standing debate on cisplatin-versus carboplatin-based chemotherapy in the treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2007;2:96.
- Fukuda M, Ohe Y, Kanzawa F, Oka M, Hara K, Saijo N. Evaluation of novel platinum complexes, inhibitors of topoisomerase I and II in non-small cell lung cancer (NSCLC) sublines resistant to cisplatin. Anticancer Res 1995;15:393–8.
- Ohe Y, Nakagawa K, Fujiwara Y, Sasaki Y, Minato K, Bungo M, . In vitro evaluation of the new anticancer agents KT6149, MX-2, SM5887, menogaril, and liblomycin using cisplatin- or adriamycin-resistant human cancer cell lines. Cancer Res 1989;49:4098–102.
- Shibayama T, Hotta K, Takigawa N, Tada A, Ueoka H, Harita S, . A phase I and pharmacological study of amrubicin and topotecan in patients of small cell lung cancer with relapsed or extensive-disease small cell lung cancer. Lung Cancer 2006;53:189–95.
- Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, Gomi K, . Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol 2008;26:5401–6.
- Kato T, Nokihara H, Ohe Y, Yamamoto N, Sekine I, Kunitoh H, . Phase II trial of amrubicin in patients with previously treated small cell lung cancer (SCLC). Proc Am Soc Clin Oncol 2006;24:abstr 7061.
- Kaneda H, Kurata T, Tamura K, Uejima H, Nakagawa K, Fukuoka M. A phase I study of irinotecan in combination with amrubicin for advanced lung cancer patients. Anticancer Res 2006;26:2479–85.
- Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, . Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 2009;27:2604–14.
- Saito Y, Maekawa K, Ozawa S, Sawada J. Genetic polymorphisms and haplotypes of major drug metabolizing enzymes in East Asians and their comparison with other ethnic populations. Curr Pharmacogenomics 2007;5:49–78.
- Theodoulou M, Hudis C. Cardiac profiles of liposomal anthracyclines: Greater cardiac safety versus conventional doxorubicin? Cancer 2004;100:2052–63.
- Noda T, Watanabe T, Kohda A, Hosokawa S, Suzuki T. Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs. Invest New Drugs 1998; 16:121–8.
- Yana T, Negoro S, Takada Y, Yokota S, Fukuoka M. Phase II study of amrubicin (SM-5887), a 9-amino- anthracycline, in previously untreated patients with extensive stage small-cell lung cancer (ES-SCLC): A West Japan Lung Cancer Group Trial. Proc Am Soc Clin Oncol 1998;17:450a(abstr 1734).
- Ohe Y, Negoro S, Matsui K, Nakagawa K, Sugiura T, Takada Y, . Phase I-II study of amrubicin and cisplatin in previously untreated patients with extensive stage small cell lung cancer (ED-SCLC). Proc Am Soc Clin Oncol 2004;23: 664(abstr 7206).
