Abstract
Background. To study the impact of inflammatory cells in a clinically well-defined cohort of women with node-negative breast cancer in a nested case-control study design. Material and methods. The cohort was comprised of 190 women who died from breast cancer and 190 women still alive at the date of death for the corresponding breast cancer patients were used as controls. The inclusion criteria included; a tumour size ≤ 50 mm, no lymph node metastases and no initiation of adjuvant chemotherapy. Immunohistochemical stainings for CD3, CD4, CD8, FoxP3, CD20, tryptase and CD68 were performed on TMA blocks, evaluated and correlated to each other and to age, tumour size, histological grade, ER, PgR, Ki67 and cyclin A. Results. There was no difference regarding the amount or content of inflammatory cells in the cases compared to controls. T- and B-cells were highly correlated to each other but these cell types correlated to a lesser extent to macrophages and not at all to mast cells. A weak tendency of correlations between all the subsets of inflammatory cells and histological grade, Ki67 and cyclin A was observed, although a negative correlation was seen for mast cells. Conclusion. The amount or content of inflammatory cells in invasive breast cancer did not appear to influence death in node-negative breast cancer.
Inflammatory cells have gained a renewed interest in breast cancer research due to both our increased understanding of their role in tumour development and our increased ability to differentiate between cell types. Leukocytes affect tumour growth, macrophages are known to have several pro-tumoural functions, and genes associated with leukocyte and macrophage infiltration in certain “molecular signatures” predict a worse prognosis [Citation1]. Mast cells may contribute to tumour growth and metastases by releasing histamine, proteases and leukotrienes. Little is known about mast cells in breast cancer and only a few studies have indicated that mast cells may be involved in breast tumour pathogenesis [Citation2–4]. The amount of mast cells is generally higher in breast cancer tissue than in the benign breast [Citation5,Citation6]. In addition, we have previously, in contrast to others [Citation3,Citation4], found that an increased number of mast cells in breast cancer tissue was associated with a better prognosis [Citation7]. The immune response to tumours is mainly mediated by different T-cell populations. T-cells present an important immunological response in tumour growth in the early stages of cancer, but become suppressive CD4(+) and CD8(+) T regulatory cells (T-regs) after chronic stimulation and interaction with tumour cells, thus promoting rather than inhibiting cancer development and progression. T-regs are identifiable by the marker protein FoxP3, however how exactly these T-cells act in breast cancer tissue is largely unknown and only a number of studies on breast cancer have been reported [Citation8,Citation9]. Nonetheless, high levels of T-regs have been reported in peripheral blood, lymph nodes and tumour specimens from patients with different cancer types [Citation10,Citation11].
Our aim is to study the impact of inflammatory cells in a clinically well-characterised set of cases and controls nested within a population-based cohort of early breast cancer.
Material and methods
Patients
The patients were selected from women diagnosed with breast cancer between 1993 and 2004 and registered to the Uppsala-Örebro Breast Cancer Clinical Database. The database is continuously updated from the Swedish Cancer Register with a completeness of over 98% for breast cancer [Citation12]. We performed a nested case-control study among women that had a tumour size ≤ 50 mm, no lymph node metastases and who had not undergone adjuvant chemotherapy (n = 900). Potential cases were women who had died from breast cancer and all eligible cases were selected. Eligible controls were patients who were alive at the time of death of the corresponding breast cancer case/patient. Patient information was obtained from the Uppsala-Örebro Breast Cancer Clinical Database and the National Register for Cause of Death. Two hundred and forty cases were identified and one control was selected for each case. Of these, 50 women did not, on reviewing of patient documents or due to missing tumour blocks, meet the requirements for inclusion and were excluded: 26 women (5.5%) had new/contra lateral or locally advanced breast cancer, no paraffin blocks were found for 12 patients (2.5%), six patients (1.5%) had a non-breast cancer cause of death, four patients (0.8%) had distant metastases at diagnosis, one patient (0.1%) had received adjuvant chemotherapy and one patient (0.1%) had not undergone breast surgery. Patient characteristics are shown in .
Table I. Patients’ characteristics.
Methods
Hematoxylin-eosin (H&E) sections were reviewed and the histological grade was reclassified according to the Elston-Ellis grading system [Citation13] by one author (R-M A). H&E sections from paraffin blocks from the primary tumours were used to define representative areas from which TMAs consisting of two to four cores (1 or 3mm diameter, respectively) were constructed from each tumour; 3–4 μm thick sections were cut from array blocks and transferred to glass slides. Estrogen (ER) and progesterone (PgR) receptors (W Z), Ki67 and cyclin A and HER2 were analysed for each tumour, as previously described [Citation14].
Immunohistochemistry
The TMA sections were processed in an automatic immunohistochemistry staining machine (Autostainer; Dako, Sweden). Antigen retrieval was done in target retrieval solution (TRS) citrate buffer pH6 (S2369), TE buffer pH9 (S2367) or Dakos PT-Link with buffer pH9 (K8012) (depending on the current antibody) in a microwave oven for 10 min at 750 W and for 15 min at 350 W. The monoclonal antibodies employed were: Tryptase (MAB1222, 1:100, Chemicon), CD68 (PG-M1, 1:200, DAKO), CD20 (M0740-L26, 1:1000, DAKO), CD3 (NCL- L-CD3-PS1-PS1, 1:100, Novacastra), CD4 (IR649, ready-to-use K8000, DAKO), CD8 (M7103-C8/144B), 1:100, DAKO, FoxP3 1:100 (Abcam ab22510) cyclin A (NCL-Cyclin A, 1:100; NovoCastra Laboratories), Ki67 (1:200, M7240; Dako), ER (NCL-ER-6F11, 1:150; NovoCastra Laboratories), and PgR (NCL-PGR, 1:100; NovoCastra Laboratories). Immunostainings were analysed via DAKO Cytomation envision/HRP kit K5007.
Evaluation of immunohistochemistry
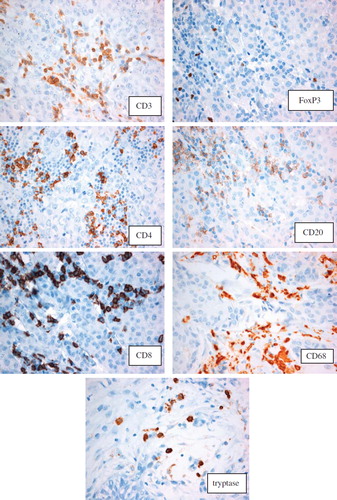
The tryptase (cytoplasmic staining for mast cells), CD68 (cytoplasmic staining for macrophages), CD20 (membraneous staining for B-cells), CD3 (membraneous staining for T-cells), CD4 (membraneous staining for T-helper cells), CD8 (membraneous staining for T-suppressor cells) and FoxP3 (nuclear staining for T-regs) TMAs were analysed by one of the authors (BL). All of the TMAs for CD68 were also analysed by another author (R-M A). The number of inflammatory cells were counted in four high-power fields (40 × objective) per TMA tissue core available from each tumour (1 to 4) and then divided by the number of cores. Each value is a mean value of each inflammatory cell type in four high-power fields (40 × objective).
Statistical analysis
Conditional logistic regression analysis was performed to estimate odds ratios (ORs) and confidence intervals (CIs) using the PHREG procedure in SAS (SAS Institute Inc, Cary, NC, USA). Mean values of CD3, CD4, CD8, CD20, FoxP3, CD68 and tryptase were analysed in univariate models. Different cut-off values were tested in explorative analyses. Since histological grade and Ki-67 were highly correlated with mean CD3 in this study, multivariate analysis including both these covariates simultaneously was considered not to be appropriate. In addition, models adjusted for age and tumour size were used.
Results
Staining results
Of 190 case-control sets analysed on the TMAs, data were missing from five sets (5/190 = 3%), for tryptase, 12 sets (6%) were not available, for CD68, four sets (2%) for CD3, five sets (3%) for CD20 and 11 sets (6%) for CD8, CD20 and FoxP3. Loss of some data occurred due to having too little evaluable tumour tissue on the TMA.
The distribution of each inflammatory cell type showed no statistical differences between the cases and the control group (). A tendency towards a higher mean value of inflammatory cells in the control group of TMAs was observed, with the exception of macrophages and FoxP3 + T-cells, although this was not statistically significant. Higher levels of CD8 compared to CD4 positive T-cells were also observed. The different stainings are presented in .
Table II. Descriptive statistics. Mean, median, minimal and maximal values of the different cells types presented.
Correlation of the different inflammatory cells to each other and to other clinicopathological parameters
The number of inflammatory cells was strongly positively correlated to each other (Spearman correlation) with the exception of the amount of mast cells (tryptase +) compared to macrophages (CD68 +), where no significant correlation was observed. Spearman's correlations are presented in .
Table III. Spearman correlation. Each parameter was correlated to each other.
All of the inflammatory cell types except mast cells were significantly (p < 0.05) positively correlated to the values of Ki67, cyclin A and histological grade, although the r-values were modest.
The subsets of T-cells positive for CD3, CD4 and FoxP3 were negatively correlated to ER- and PgR-expression. Tryptase positive mast cells were negatively correlated to tumour size, age at diagnosis, Ki67, histological grade and cyclin A, but positively correlated to PgR.
CD68 positive macrophages were negatively correlated to ER and positively correlated to tumour size, Ki67, histological grade and cyclin A (). HER2 status was dichotomised and a correlation could therefore not be calculated.
Risk of breast cancer death
No statistically significant OR was discovered in terms of the number of inflammatory cells in the tumour tissue with regards to risk of death due to breast cancer in the cases compared to the controls. Data is presented in .
Table IV. Conditional logistic regression. Case-control study. End point: breast cancer death. Models adjusted for tumour size and age at diagnosis. Different cut-off values were tested in explorative analyses and cut-off at 50 percentile is presented.
Discussion
We were unable to show any quantitative differences in the inflammatory infiltrate between the case and control groups. An interesting finding was however, the correlation between a high density of inflammatory cells, where the strongest factor was the presence of CD3 positive T-cells, to adverse prognostic markers: Ki67, cyclin A and histological grade. However, the association – although statistically significant (p < 0.05) – was modest.
Macrophages have been identified as adverse prognostic factors but we were unable to confirm these results [Citation15]. Tumour-associated macrophages have, however, also been found to correlate with good clinical outcome [Citation16,Citation17]. It is possible that macrophages in the tumour microenvironment have dual functions. Other studies on macrophages have, in contrast to our study, included women with lymph node metastases. This could imply that macrophages are part of tumour progression at later stages in the tumour development and not involved in early carcinogenesis and this could explain why we do not detect such differences in the present study.
In addition, subgroup analyses of ER + and ER- patients were not investigated in the present study, but it is possible that the amount of inflammatory cells affect outcome only in ER negative patients [Citation18,Citation19].
The cases and controls did not differ in terms of the total amount of mast cells, but we were able to confirm that mast cells were negatively associated to other factors related to a worse prognosis, such as increased tumour size, age and proliferation markers [Citation7]. Our results are in contrast to a number of other studies, although these investigations were based on smaller patient cohorts [Citation3,Citation4].
The strong correlations between the different subsets of T-cells positive for CD3, CD4, CD8 and FoxP3 were expected since these markers merely identify the same types of cells. A high correlation between CD3 positive T-cells and CD20 positive B-cells was however somewhat unexpected and this finding could tentatively/possibly be explored further. Interestingly, more CD8 than CD4 positive T-cells were present in total, which is not the case in reactive inflammatory infiltrates, where CD4 positive cells are more common than CD8 positive cells. This implies an increased presence of cytotoxic CD8 positive T-cells. Tumour-infiltrating CD8(+) T lymphocytes have been shown to have a favourable effect on patients’ survival [Citation18] but CD8 positive cells in our study did not appear to influence the death rate due to breast cancer. We therefore believe that these subsets of different T- and B-cells do not have a dominant role in breast cancer prognosis.
Heterogeneity in the investigated tumour material may affect the strength of a prognostic marker or even determine whether it is prognostic or not [Citation20] but our own unpublished data on the surrounding cells in Hodgkin lymphoma has shown a good correlation between the amount of Tregs in TMAs compared to whole tissue sections. We have however, not made comparisons to whole tissue sections in this study.
We used a well-characterised cohort with complete follow-up from a population-based register with high coverage. The exclusions from the case and the control series was equally large and due to similar reasons in both groups. Our data should thus be representative and valid for the whole cohort. The statistical power was also appropriate to detect clinically relevant differences.
Conclusions
Our negative results indicate that future studies of inflammatory cells in breast cancer must extend further than just analyses based wholly on cell counts, to include further characterisation of cell functions and interactions between tumour and stroma, in order to be fruitful in gaining a deeper understanding of the processes giving rise to breast cancer.
Declaration of interest: The author(s) declare that they have no conflict of interest.
References
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, . Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008;14:518–27.
- Dimitriadou V, Koutsilieris M. Mast cell-tumor cell interactions: For or against tumour growth and metastasis? Anticancer Res 1997;17:1541–9.
- Ranieri G, Ammendola M, Patruno R, Celano G, Zito FA, Montemurro S, . Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol 2009;35:115–20.
- Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Report 2010;23:615–9.
- Kashiwase Y, Morioka J, Inamura H, Yoshizawa Y, Usui R, Kurosawa M. Quantitative analysis of mast cells in benign and malignant breast lesions. Immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Int Arch Allergy Immunol 2004;134:199–205.
- Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer 2003;107:159–63.
- Amini RM, Aaltonen K, Nevanlinna H, Carvalho R, Salonen L, Heikkila P, . Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer 2007;7:165.
- Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, . FOXP3 expression and overall survival in breast cancer. J Clin Oncol 2009;27:1746–52.
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, . An evaluation of the clinical significance of FOXP3(+) infiltrating cells in human breast cancer. Breast Cancer Res Treat 2011;127:99–108.
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, . Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756–61.
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9:606–12.
- Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
- Ahlin C, Zhou W, Holmqvist M, Holmberg L, Nilsson C, Jirstrom K, . Cyclin A is a proliferative marker with good prognostic value in node-negative breast cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2501–6.
- Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, . Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat 2011;128:703–11.
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol 2002;196:254–65.
- Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, . High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer 2008;98:1118–24.
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, . Tumor-infiltrating CD8 + lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949–55.
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2011;127:99–108.
- Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: Progress in the discovery and validation of cancer biomarkers. J Clin Oncol 2008;26:5630–7.