Abstract
Aims and background. To describe feasibility, tolerability and clinical outcomes of stereotactic body radiation therapy (SBRT) in the treatment of adrenal metastases in 34 consecutive cancer patients. Material and methods. Between March 2004 and July 2010, a total of 34 consecutive patients, accounting for 36 adrenal metastatic lesions, were treated with SBRT. SBRT treatments were delivered by a Linac Varian 600 with microMLC (3DLine, Elekta, Stockholm, Sweden) and a Linac ELEKTA Precise (Elekta). All 34 patients were clinically and radiologically evaluated during and after completion of SBRT. Following outcomes were taken into account: best clinical response at any time, local control, time to systemic progression, time to local progression, overall survival and toxicity. Survival was estimated by the Kaplan-Meier method and factor potentially affecting outcomes were analyzed with Cox regression analysis. Results. Total RT doses ranged from 20 Gy in 4 fractions to 45 Gy in 18 fractions (median dose: 32 Gy; median number of fractions: 4). All doses were prescribed to the 95% isodose line. No cases of Grade ≥ 3 toxicity were recorded. At a median follow-up time of 41 months (range, 12–75) 22 patients were alive. Three of 28 lesions (11%) showed complete response, 13/28 (46%) partial response, 10/28 (36%) stable disease and 2/28 (7%) progressed in the treated area. Local failure was observed in 13 cases. Actuarial local control rates at one and two years were 66% and 32%, respectively. Median time to local progression was 19 months. Median survival was 22 months. Conclusion. SBRT in adrenal gland metastasis is feasible without significant acute and late toxicities, with a good rate of local control. New SBRT fractionation schemes and the possibility to combine new systemic approaches should be investigated in order to further increase local control and reduce systemic disease progression.
Adrenal gland metastases can occur from various types of extra-adrenal primary cancers, although the most frequent primary tumor is non-small cell lung cancer (NSCLC) [Citation1].
In the last two decades, a global increase in overall survival and the widespread use of abdominal computed tomography (CT) staging and follow-up imaging in metastatic patients has permitted to detect an increased rate of adrenal gland metastases [Citation2,Citation3]; therefore the presence of an isolated adrenal gland metastasis is now more frequent in clinical routine.
In these patients, surgery can be often considered a valid treatment option. Several surgical experiences have evaluated the role of adrenalectomy in improving survival for various types of controlled primary tumors [Citation4,Citation5]. Open surgery has been frequently the treatment of choice for these patients and, more recently, laparoscopic adrenalectomy has been introduced in the practice with the advantage of a minimally invasive approach [Citation6,Citation7]. Other local ablative treatments, such as radiofrequency seem to be comparable to surgery in terms of oncologic efficacy in subsets of carefully selected patients [Citation8].
In the scenario of single or oligo-metastatic focal disease, radiotherapy (RT) could have a significant role not only in symptom palliation, but also in local control. Highly conformal radiation therapy, such as stereotactic radiosurgery, stereotactic body radiotherapy (SBRT) or similar techniques are able to deliver biologically equivalent doses dramatically higher than palliative RT, and in sites, such as the lung, SBRT has proved to be as effective as surgery, with the advantage of decreased morbidity and the potential to deliver ablative treatment at lower costs and on an outpatient basis [Citation9].
To date, few data regarding the role of SBRT in adrenal metastases have been published. The present study describes retrospectively our experience in treating adrenal metastases with SBRT, with regards to response, local control, time to progression and toxicity.
Material and methods
Between March 2004 and July 2010, a total of 36 adrenal gland metastatic lesions, in 34 consecutive patients, were treated at the Humanitas Clinical Institute (ICH) and at the University of Turin. Records of all 34 patients were retrospectively analyzed.
Patients were eligible for this trial if they had pathologically confirmed stage IV cancer of any histology with distant metastases. Additionally, patients were required to be 18 years or older, have a life expectancy of > 3 months, Eastern Cooperative Oncology Group performance status of ≤ 2, normal marrow and organ function, and no prior RT to involved tumor sites. Baseline CT-scans of the chest, abdomen, and pelvis as well as bone scan or, preferably, positron emission tomography (PET) scan were obtained no more than two months before treatment. Patients with a life expectancy < 3 months and/or with any serious disease contraindication or radiation therapy were excluded. Other coexisting malignancies, uncontrolled intercurrent illness, active infectious processes, and exudative, bloody, or cytologically malignant effusions excluded patients from the trial. Additionally, patients were excluded if they had received any systemic chemotherapy during RT, although hormonal therapy was allowed. No prior radiation therapy to the targeted area was allowed.
Written informed consent was obtained from all patients before beginning SBRT.
All patients were immobilized in a body cast combined with a vacuum pillow inside a stereotactic frame or in frameless body-fix systems; CT-scan of upper abdomen was then acquired for every patient and clinical target volume (CTV) was then defined from an isotropical expansion of 3 mm from gross tumor volume (GTV). GTV was defined on CT images that were compared to previous diagnostic CT-scans or, when available, especially for recent patients, with contrast media enhancement. The CT was acquired in free breathing without contrast medium. The slice thickness was 3–5 mm. Starting in 2009, a three-phase contrast medium CT was also performed just after the first CT. In all cases, dose calculation was performed on the CT without contrast medium. The contoured organs at risk (OAR) were spinal cord, kidneys and liver. No defined dose-constraints were adopted and in each case the strategy was to try to maintain the dose as low as possible. The approval of each plane of treatment was based on single physician decision.
In the period of study, the gated CT during simulation to include organ motion was not available. Thus, no personalized margins were defined for any of the patients and, in accordance with the international indications of ICRU, we isotropically expanded the CTV to the planning target volume (PTV) including population-based internal target volume (ITV) and setup margin (SM) with a cumulative standard margin of 5 mm. The total administered doses ranged from 20 Gy in 4 fractions to 45 Gy in 18 fractions.
SBRT was delivered by a Linac Varian 600 (Varian, Palo Alto, CA, USA), with microMLC (3DLine, Elekta, Stockholm, Sweden), and an Elekta Precise LINAC (Elekta). All plans were made using dynamic arc therapy, with five to seven non-coplanar arcs of 30°. Isocenter position was detected using a body-frame localization of 3D-line. Prior to each treatment fraction a 2D-2D match based on bony anatomy was performed to best reposition the patient. No patient based pretreatment quality assurance was performed as dynamic arcs were used. However, before starting SBRT a huge study was made to verify the dosimetry of small fields.
Systemic therapy, such as chemotherapy, was administered until one month before SBRT and/ or two months after SBRT, but not during RT treatment.
All patients were clinically evaluated weekly during the RT course and then after the completion of the treatment with a predefined follow-up schedule: for the first year every three to four months (clinical exam and CT-scans) and for the second and third year every six months (clinical exams and CT-scans).
Toxicity was scored according to the Common Terminology Criteria for Adverse Events (CTC AEv3.0).
The clinical response was defined as the best response at any time after SBRT during follow-up, using the Response Evaluation Criteria in Solid Tumors (RECIST) [Citation10]. According to RECIST criteria, local control was defined as the absence of progression [complete or partial remission and stable disease (SD)] in irradiated adrenal gland metastases after SBRT.
Observation time started after the last fraction of SBRT. Study endpoints were: response, local control, time to local progression, time to systemic progression and overall survival. Secondary endpoints were acute and late toxicity.
Descriptive statistics were summarized as frequencies and percentages for categorical variables and as median and range for continuous variables. Distribution of local control, PFS and OS were estimated using the Kaplan-Meier method. Multivariate analysis was carried out using the Cox proportional hazards model, reported with hazard ratio (HR) and 95% confidence interval (CI), and a significance level of 0.05 was used for covariate entry. Two-tailed p-value of < 0.05 was considered statistically significant. SAS 9.2 release was used for all statistical analyses.
Results
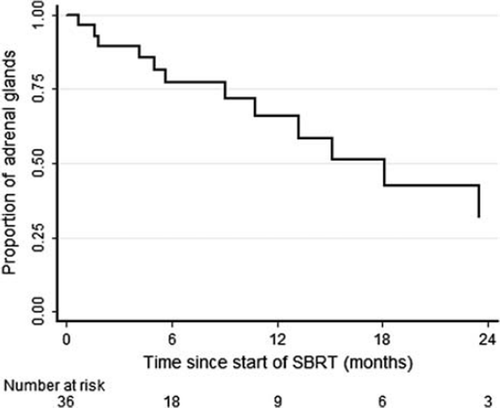
Patients’ characteristics are shown in detail in . The median interval from primary cancer diagnosis to the occurrence of adrenal metastasis was 12.5 months (range, 0–95 months). Prior to receiving SBRT, 12/34 patients had metastases to other organs: lung/mediastinum (n = 7), brain (n = 2), bone (n = 1), lymph node (n = 1 axillary) and liver (n = 1). All patients underwent SBRT with SD (for a minimum duration of six months) in other metastatic sites. However, adrenal metastasis was the only metastatic site in progression at the time of SBRT. The CTVs ranged from 8.1 cm3 to 579.4 cm3, with a median volume of 44.0 cm3. As shown in , SBRT doses ranged from 20 Gy in 4 fractions to 45 Gy in 18 fractions with a median cumulative dose of 32 Gy and a median number of fractions of 4. The biological equivalent dose (BED) for the tumor, assuming α/β = 10 Gy, ranged from 30 Gy10 for the 5 Gy × 5 days schema to 56.3 Gy10 for the 2.5 Gy × 18 days. All doses were prescribed to 95% isodose. Minimum follow-up time was 12 months (range, 12–75, median of 41 months). All patients were assessable for acute toxicity. No patient developed Grade ≥ 3 gastrointestinal, hepatic, renal, or dermatologic toxicity. Grade 2 nausea was recorded in two (6%) patients. Twenty-eight of the 34 patients were therefore evaluable for late toxicity. Late gastrointestinal, renal, or hepatic or dermatologic toxicity was not observed.
Table I. Description of general patient characteristics, site of primary tumor, adrenal metastases and treatment characteristics.
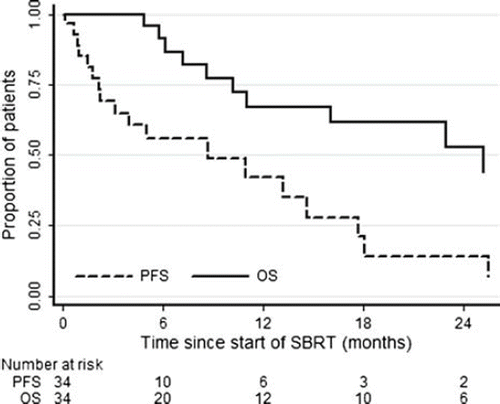
Twenty-eight of 34 patients were definitively evaluable for primary and secondary study endpoints. Data were not completely available to assess results of six patients lost to follow-up. At the time of definitive analysis, 22/28 were alive. Clinical response was evaluated on CT. PET total body scan was also requested during follow-up in 3/34 patients (9%). The best clinical response was adopted to establish clinical result after RT: 3/28 (11%) irradiated lesions showed complete response (CR), 13/28 (46%) lesions showed a partial response (PR), 10/28 (36%) had SD, 2/28 (7%) had progression in treated area. Actuarial local control rates at one and two years, estimated by means of Kaplan-Meier method, were 66% and 32%, respectively. At the time of definitive analysis, 22 patients were alive and in 18 there was systemic progression. During follow-up, 13 cases of 28 (46%), failed locally at treated sites. Median time to local progression was 19.7 months (), while median time to systemic progression 10.3 months (). Median overall survival was 22.8 months (). At two years 53% of patients are alive, 32% in local control and 14% without distant metastases.
Discussion
From several phase II studies, results of SBRT for isolated lung, or for isolated or few metastases are comparable to surgical series in selected patients [Citation9,Citation11–15]. Specific selection criteria to offer SBRT to patients with various isolated or oligometastatic tumors have been recently proposed. They included: controlled primary, favorable histology, limited metastatic disease, metachronous appearance of metastasis, young age and high performance status of the patient [Citation15]. In current experience, we recruited patients for SBRT to adrenal metastasis when Karnovsky performance status is > 70 and when primary tumor is controlled, in absence of other active metastases. In our group of 34 patients, the last two points are usually documented with the last CT and or FDG-PET before SBRT.
In general, increased median and overall survival has been demonstrated for resection of clinically isolated adrenal metastases when compared to nonsurgical therapy including radiofrequency ablation, external beam RT arterial embolization, chemical ablation and cryoablation [Citation16]. In the group of non-surgical approaches, technological advances in radiation planning and delivery have now rapidly expanded this non-invasive modality as another potential form of local therapy, also for adrenal metastasis. describes and compares the characteristics of studies published on the subject of SBRT for adrenal metastasis. SBRT for adrenal metastases has been employed and reported in few series and there is no consensus regarding prescription doses. In our series, doses ranged from 20 Gy in 4 fractions to 45 Gy in 18 fractions. The reason for the dose prescription variability depended on the absence of consensus or defined guidelines for radiation treatment of adrenal gland metastases during the study period in both institutes, mainly due to the low frequency in clinical practice. Thus, the dose was prescribed based on single physician decision. Clearly, this large dose range, concerning variability of total dosage and number of fractions, is a reason for criticism because of the lack of homogeneity in the population under study. However, the small number of experiences published on the subject report the same variability in dose scheduling [Citation17–20].
Table II. Description and comparison of the main characteristics of the studies published on this subject.
Concerning doses and fractionation schedules, the more homogeneously treated group was the one included in the report by Florence University [Citation21]. However, although in the majority of patients (40 of 48 cases), the prescription dose was 36 Gy in 3 fractions with multi-fraction stereotactic radiotherapy, eight patients were treated with single-fraction stereotactic radiosurgery.
A limitation of the present study is that we retrospectively analyzed a population of patients with adrenal metastasis from different primary tumors. Although 22 of 36 lesions were from NSCLC, we included in the study adrenal metastatic patients with other primary malignancies. As well as in other published experiences, the choice to report on a miscellaneous population was determined by the general lack of data and by the difficulty in recruiting a sufficiently large and homogeneous population of adrenal gland metastasis patients.
Considering that systemic therapy could constitute a bias in assessing local results, we underlined that no chemotherapy was administered concomitantly with SBRT course in our study population. Instead, systemic therapy was prescribed only at disease progression.
Of the 28 patients in our study with complete data for assessing definitive response 22 were alive at the time of definitive analysis; excluding two cases of progressive disease, the remaining 26 patients showed local control (CR or PR or SD) after treatment with a time to local progression in irradiated gland exceeding 19 months. Of these 28 patients assessed for long follow-up, 18 showed systemic progression with a time to systemic progression of around 10 months.
Chawla et al. reported in their experience a single case of CR, 15 PR, four SD, and four progressive diseases [Citation17].
Optimal local control was observed by Oshiro et al. reporting only a recurrent local lesion in one of the eight patients with death caused by cancer progression. In the same report, none of the irradiated patients experienced severe toxicity related to RT [Citation18].
In the series of 48 patients reported by Florence University, with a median follow-up time of 16.2 months, the actuarial one- and two-year local control rate was 90%. All 48 patients experienced distant failures and in two cases a local failure, with a median interval to local failure of 4.9 months. The actuarial one-year disease control rate was 9%. OS rates at one- and two-years were 39.7% and 14.5%, respectively [Citation21].
In our experience overall survival at two years was 53% of patients, actuarial local control at one and two years was 66% and 32%, respectively, and distant metastasis-free survival 14%. Although after SBRT clinical local control was achieved in more than 90% of treated lesions, the rate of systemic failure still remains high (64%; 18/28 cases), in tune with the other published experiences. Median time to local failure was longer than 19 months while median time to systemic progression was around 10 months.
SBRT was feasible and well tolerated. Significant acute and late side effects were not observed and clinical local control was achieved in more than 90% of cases with response duration longer than 19 months. However, systemic failure rates strongly affect survival in adrenal gland metastasis patients.
In the current retrospective study we analyzed data of “conformal SBRT” treatments. The intensity modulated approaches using either static fields, dynamic arcs or protons are superior to the other conformal solutions [Citation22]. For their simplicity in daily clinical practice and safety in abdominal lesion irradiation, volumetric intensity modulated arc therapy (VMAT) or similar approaches [Citation23] should be considered a potential option for radiation treatment of precision in these patients.
Another interesting issue is the BED of prescribed doses in our patients. A BED greater than 100 Gy was recently suggested in SBRT for early NSCLC to optimize clinical results [Citation20]. Analyzing retrospectively the doses of our SBRT treatments, the BED value ranged from 30 Gy10 for the 5Gy × 5 days schema to 56.3 Gy10 for the 2.5 Gy × 18 days. The low BED depended on the fact that the study started in early 2004, when image guidance with Cone beam CT was not available, as well as the gated CT to include organ motion and thus, we preferred to start using relatively low total doses, to preserve surrounding organs at risk. We can hypothesize that higher equivalent doses to adrenal gland metastases, especially from a NSCLC primary tumor, could be more efficient than ours to increase clinical benefits of SBRT.
Thus, new SBRT techniques with intensity-modulated radiation therapy (IMRT)/VMAT and image-guided radiation therapy (IGRT), exploring more aggressive and effective fractionation schemes, and also the possibility to combine radiation with new systemic approaches, need further studies to optimize the duration of local control and the development of systemic progression.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lam KY, Lo CY. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101.
- Mayo-Smith WW, Boland GW, Noto RB, Lee MJ. State-of-the-art adrenal imaging. Radiographics 2001;21:995–1012.
- Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, . 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med 2004;45:2058–62.
- Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis Cancer 1998;82:389–94.
- Uberoi J, Munver R. Surgical management of metastases to the adrenal gland: Open, laparoscopic, and ablative approaches. Curr Urol Rep 2009;10:67–72.
- Sarela AI, Murphy I, Coit DG, Conlon KC. Metastasis to the adrenal gland: The emerging role of laparoscopic surgery. Ann Surg Oncol 2003;10:1191–6.
- Duh QY. Laparoscopic adrenalectomy for isolated adrenal metastasis: The right thing to do and the right way to do it. Ann Surg Oncol 2007;14:3288–9.
- Hawksworth J, Geisinger K, Zagoria R, Kavanagh P, Howerton R, Levine EA, . Surgical and ablative treatment for metastatic adenocarcinoma to the liver from unknown primary tumor. Am Surg 2004;70:512–7.
- Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: Stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys Epub 2011 Jun 1.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . for the European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–16.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, . Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677–82.
- Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, . Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72:398–403.
- Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, . Tolerance of organs at risk in small volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 2003;56:126–35.
- Rubin P, Brasacchio R, Katz A. Solitary metastases: Illusion versus reality. Semin Radiat Oncol 2006;16:120–30.
- Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol 2006;16:77–84.
- Duh QY. Resecting isolated adrenal metastasis: Why and how? Ann Surg Oncol 2003;10:1138–9.
- Chawla S, Chen Y, Katz AW, Muhs AG, Philip A, Okunieff P, . Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys 2009;75:71–5.
- Oshiro Y, Takeda Y, Hirano S, Ito H, Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol 2011;34:249–53.
- Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187:245–51.
- Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: A retrospective review of a noninvasive therapeutic strategy. Future Oncol 2011;7:145–51.
- Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys Epub 2011 Feb 5.
- Scorsetti M, Mancosu P, Navarria P, Tozzi A, Castiglioni S, Clerici E, . Stereotactic body radiation therapy (SBRT) for adrenal metastases: A feasibility study of advanced techniques with modulated photons and protons. Strahlenther Onkol 2011;187:238–44.
- Zhang J, Yang F, Li B, Li H, Liu J, Huang W, . Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305–16.