Abstract
Background. Consensus is that patients with locally advanced rectal cancer (LARC) should receive long-term chemoradiotherapy (CRT) before surgery. With the intent to offer the patients intensified concomitant chemotherapy (CT) to improve outcome and to assess tolerability and toxicity of oxaliplatin (Ox) a phase I trial of high dose pelvic radiotherapy (RT), fixed dose of oral UFT/l-leucovorin and increasing doses of weekly Ox were performed. Methods. Pelvic RT with 48.6 Gy/27 fractions was given to the primary tumour and the regional lymph nodes and a concurrent boost of 5.4 Gy/27 fractions with a final boost of 6 Gy/3 fractions was given to the gross tumour volume (GTV) (60 Gy/30 fractions). Concurrent with RT patients received a daily dose of UFT 300 mg/m2 plus fixed dose l-leucovorin 22.5 mg 5/7 days and increasing weekly doses of Ox with 10 mg/m2/week from a start dose of 30 mg/m2/week to a maximum of 60 mg/m2/week. In addition, before and after CRT the patients received one course of TEGAFOX (UFT 300 mg/m2 with l/leucovorin 22.5 mg Days 1–14 and Ox 130 mg/m2 given on Day 1). Surgery was planned at least six weeks after the completion of the CRT. Results. From May 2005 to March 2009, 18 patients with LARC (16 primary, two recurrent) were included in this phase I trial. Toxicity was low with only 5–17% grade 3–4 toxicity. Fifteen patients (83%) were operated (14 R0 resection and 1 R1 resection) after completion of CRT. Five (33%) patients had a pathological complete response (ypCR). When ypCR was combined with yp few residual cells, the rate was 60%. Thirteen patients are still alive December 2011. Conclusion. Preoperative high-dose RT and concomitant UFT with increasing doses of Ox up to 60 mg/m2/week was feasible with low toxicity, high ypCR rates and promising OS in patients with non-resectable LARC.
Surgery is the primary, potentially curative, treatment for localised rectal cancer (RC) and the aim is to obtain a R0 resection in order to reduce the local recurrence rate and to prolong survival. In the last decade the introduction of total mesorectal excision (TME) with a multidisciplinary approach combining pre- or postoperative radiotherapy (RT) or chemoradiotherapy (CRT) has contributed to a low local recurrence rate of around 10% and five-year survival of 60% [Citation1,Citation2].
The 10–15% true non-resectable RC are the most advanced T4 tumours fixed to the pelvic wall or with invasion of nearby internal organs, such as the prostate, uterus or the bladder. These tumours have traditionally been treated with conventionally fractionated (1.8–2.0 Gy/fraction) long-course preoperative RT to a dose of 45–60 Gy to obtain control of local advanced disease and to induce tumour shrinkage to allow subsequent radical surgery which has been possible in 40–80% of the patients leading to possible long-term survival in 20–30% of these patients [Citation3]. Delayed surgery after four to eight weeks was then performed if the tumour was down-sized/staged or was no longer clinically fixed. Essentially there are two approaches to improve local control with RT: either use a higher radiation dose or use radiosensitisers as 5-fluorouracil (5-FU). Continuous venous infusion of 5-FU (CVI) is better than bolus infusion [Citation4]. UFT is an oral formulation of the prodrug tegafur and uracil. Tegafur is converted to 5-FU primarily in the liver and uracil inhibits the degradation of 5-FU [Citation5]. The plasma concentrations of 5-FU on UFT treatment mimics that of CVI [Citation6].
Oxaliplatin (Ox) in combination with 5-FU has proven efficacy in the adjuvant and metastatic setting and in addition Ox is a drug with good potential as a radiosensitiser. Early CRT studies with concomitant Ox and RT doses up to 50 Gy have produced ypCR rates in the range of 15–30% [Citation7,Citation8]. Apparently there exists a dose-response relationship and perhaps a higher radiation-dose could improve the local control [Citation9–11].
A phase I and subsequent phase II trial with preoperative high-dose RT (60 Gy in 30 fractions using concomitant boost techniques) and concurrent oral UFT/l-leucovorin (Lv) to patients with non-resectable rectal cancer (primary and recurrent) showed major regression in most patients [Citation5,Citation12]. Here we present a phase I trial to investigate the feasibility of high-dose radiotherapy (60 Gy) concomitant with dose escalating weekly Ox, oral UFT and a fixed dose of l-Lv in combination with preoperative combination chemotherapy (CT) (preoperative TEGAFOX before and after CRT). Postoperative CT was not part of this protocol as the use of adjuvant CT in rectal cancer is still controversial [Citation13,Citation14]. The primary end-point was to determine the Maximal Tolerable Dose (MTD) and Dose Limiting Toxicity (DLT) of Ox and as a secondary end-point we also wanted to assess toxicity and efficacy.
Material and methods
Criteria of eligibility
All patients had biopsy-proven non-resectable (primary or recurrent) rectal adenocarcinoma (LARC). Patients were eligible if the tumour was fixed to the pelvic wall or otherwise non-resectable as judged clinically by an experienced colorectal surgeon. Patients were required to have a WHO performance status 0–2, age > 18 years and adequate bone marrow, renal and hepatic function (WBC > 3 × 109/l, platelet count > 100 × 109/l, total bilirubin level ≤ 1.5 × upper normal value, ASAT and/or ALAT ≤ 3 × upper normal value and a creatinine clearance ≥ 30 ml/min).
Exclusion criteria were extrapelvic disease, prior adjuvant chemotherapy with 5-FU within 12 months, previous therapy with UFT or Ox, previous pelvic irradiation, laparotomy within four weeks before inclusion, prior or concomitant malignant disease, or pregnant or lactating women.
Investigations and follow-up
Performance status, weight, blood samples and toxicity were evaluated at baseline, before CRT, and weekly during CRT. Pelvic magnetic resonance imaging (MRI)-scan was performed to supplement the clinical judgment of non-resectability and abdominal and thoracic computed tomography scan was performed to exclude extrapelvic disease.
Ethics
The protocol and the procedures followed were according to the Helsinki Declaration and were approved by the regional Ethical Committee and the Danish Health Authorities.
Radiotherapy
RT was delivered five days a week, once a day, to a total dose of 60 Gy in 30 fractions to the primary tumour. This was accomplished using 27 fractions of 1.8 Gy to the pelvis with a concomitant boost of 27 fractions of 0.2 Gy to the primary tumour, and a final boost of 3 fractions of 2.0 Gy.
Macroscopically gross tumour volume (GTV) included the primary tumour and other macroscopically identified tumour. GTV was defined by integrated information obtained by MRI-scan and any clinical information. The clinical target volume (CTV) was defined as GTV plus tissue harbouring potential microscopic disease including presacral and perirectal areas with lymph nodes as well as internal iliac lymph nodes. The upper border of the radiation portals was the promontorium (the junction L5-S1) and the lower was 3 cm below the primary tumour or 1 cm below the obturator foramen. If there was distal extension of the tumour to the anal verge, the perineum was included.
All patients were treated supine using high-energy megavoltage linear accelerator. A five-beam technique with concomitant boost was used. A posterior (field 1) and two lateral fields (fields 2 and 3) encompassed CTV (1.8 Gy/day) and, as concomitant boost, two lateral boost portals (fields 4 and 5) encompassed GTV with a 1-cm margin (0.2 Gy/day). The two lateral boost portals were also used for a final 6 Gy boost (2.0 Gy/day). CTV thus received 48.6 Gy in 27 fractions and GTV received 60 Gy in 30 fractions. The prescribed dose to GTV was specified according to ICRU 50 and 62 with the isodose distribution to the GTV of at least 95%. All five fields were treated in the same session.
Chemotherapy
As soon as possible after the established diagnosis patients received one cycle of preoperative chemotherapy with TEGAFOX (daily dose of oral UFT 300 mg/m2 and a fixed dose of l-leucovorin 22.5 mg Days 1–14, both divided in three daily doses, in combination with Ox 130 mg/m2 i.v. given over 30 minutes Day 1).
On Day 21 patients started radiotherapy with concurrent oral UFT 300 mg/m2/day, five days a week for 30 days, divided in three daily doses plus a fixed dose of l-leucovorin 7.5 mg (22.5 mg daily) with each UFT dose and weekly Ox. The dose of weekly Ox was escalated from 30 mg/m2 to a pre-specified maximum of 60 mg/m2/week for six weeks if no severe (grade 3 or 4) toxicity was observed (). One to two weeks after completing CRT patients received one cycle of TEGAFOX before planned surgery.
Table I. Oxaliplatin dose escalation levels and number of patients.
Toxicity
Toxicity was graded according to NCI Common Toxicity Criteria version 2.0. DLT was reached if grade 3 toxicity was observed. Cohorts of three to six patients were entered at each dose level and each cohort was evaluated for the entire combined treatment course before dose escalation was allowed. If one patient at a given dose level developed DLT, three additional patients were planned to be treated at that dose level. If zero or one out of three/six patients developed DLT the dose was escalated with 10 mg/m2/week. If two or more patients out of three or six developed DLT the MTD was reached. Antiemetic drugs were offered at every Ox dose. Antidiarrheal drugs were not offered prophylactically but could be used on demand.
Evaluation of response
The tumour response and resectability was evaluated by the investigators with an abdominal MRI-scan and digital rectal examination by an experienced colorectal surgeon four weeks after completing the treatment. Intended surgical radical resection was planned at least six weeks after the completion of the CRT in the absence of progression. The final judgement of resectability was clinical.
The operation was classified either as R0 if the resection margin examined by the pathologist was uninvolved, R1 (macroscopic radical) or R2 if macroscopic tumour remained in the operative field at the end of the surgical procedure. An experienced pathologist evaluated specimens for pathological complete response (ypCR) according to pathologic tumour regression grade (TRG) [Citation15].
Statistical evaluation
Non-parametric statistics were applied. All median values are followed by range in brackets. Overall survival curve was generated according to the Kaplan-Meier method and was calculated as the time from registration until death from any reason or the last date of follow-up. Progression-free survival time (PFS) was calculated from registration until any recurrence/progression or the last date of follow-up. PFS was updated until December 1, 2011.
Results
From May 2005 to March 2009, 18 patients (nine men and nine women) were treated according to this phase I trial. Median age was 62 years (range, 34–76 years) and median performance status was 0 (range, 0–2). Sixteen patients had primary non-resectable, T4NxM0 rectal cancer and two patients had recurrent non-resectable pelvic disease. All patients except one received the planned RT with 60 Gy in 30 fractions. A patient with ileostomy discontinued radiotherapy after 52 Gy (26 fractions) due to severe diarrhoea. The median duration of RT was 44 days (range, 38–49 days). All patients except one received the planned dose of UFT and 13 patients received the planned dose of Ox. shows the number of patients at each dose level. DLT was seen in four patients, one at dose level 0, one at level 1 and two at level 3. One patient had dose-reduction of TEGAFOX due to diarrhoea, one patient due to thrombopenia. Concomitant Ox was postponed because of severe obstipation in one patient and stent-problems in another patient who subsequently had a stoma. The latter patient did not receive the final TEGAFOX before surgery because of sign of a lung infection. Succeeding CT-scan showed progressive disease with multiple lung metastases. Furthermore, one patient did not receive the final TEGAFOX because of severe diarrhoea. shows acute toxicity grade 1–2 and grade 3 at each dose level associated with preoperative CRT. We found no obvious correlation between dose level and toxicity at dose level 0 to 2 but more grade 3 toxicities were observed for patients at dose level 3 (totally six occurrences of grade 3).
Table II. Worst toxicity according to CTC version 2.0 at each dose level. Number of patients experiencing grade 1–2 and 3 toxicity, respectively.
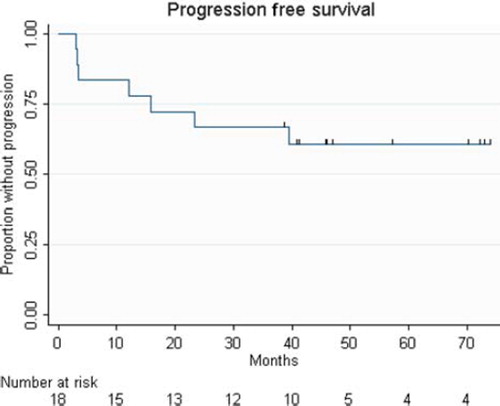
Surgery was performed in 15 patients (83%) median 60 days (range, 40–126 days) after completion of RT. There was good access to surgery for all patients. TME was performed in 13 patients and two patients had finally extensive pelvic exenterations in a specialised unit. Three patients had no surgery, one patient developed lung metastases at the time of surgery, one patient refused surgery and one patient with recurrent pelvic tumour had complete response on MRI-scan and PET/CT-scan and therefore conservative therapy was chosen. Fourteen of 15 operated patients (93%) had microscopic radical surgery (R0) and one patient had R1 resection. Five patients (33%) achieved complete pathological response (ypCR or TRG1) and all are presently alive without any sign of recurrence. Four, two and four patients obtained TRG2, TRG3 and TRG4, respectively. At the time of update (December 1, 2011) five patients had died. Three patients developed distant relapse and underwent radical metastasectomy, two for lung metastases and one for a liver metastasis. They are all disease free. One patient is still alive with recurrent disease with distant metastases. PFS for all 18 patients is shown in .
Figure 1. Kaplan-Meier curve of recurrence-free survival for 18 patients with non-resectable LARC. Patients received one course of Tegafox before and after CRT (60 Gy and UFT/l-leukovorin with increasing doses of weekly oxaliplatin) and subsequently 15 patients had surgery. Five-year PFS is 60.6% (23.3 – NR). CRT, chemoradiotherapy; LARC locally advanced rectal cancer; PFS, progression free survival.
Discussion
To the best of our knowledge, this is the first study to evaluate weekly Ox in combination with high-dose CRT (60 Gy/30 fractions with daily oral UFT/folinic acid) as preoperative therapy in patients with non-resectable LARCs. In accordance with most other phase I studies evaluating concomitant Ox in rectal cancer [Citation16] we found that a weekly dose of Ox 50 mg/m2 is tolerable and constitute the recommended dose level. For various unexpected reasons our study was altered from a multicentre study to a single institution study and this accounts for the protracted inclusion period which gave an unplanned chance to evaluate long-term efficacy in addition to toxicity.
Appropriate staging of patients with rectal cancer includes pelvic MRI-scan to evaluate the primary tumour and its relations to the mesorectal fascia and surrounding structures but also thoracic and abdominal CT-scan to exclude distant metastases. Rectal cancer can be staged according to the TNM classification but recently it has been suggested to group patients into three risk groups (low, intermediate and high, or “good”, “bad” and “ugly”) [Citation17].
Assessment of all rectal cancer at multidisciplinary team meetings with quality-control, better-quality surgery and appropriate use of preoperative RT/CRT have reduced local recurrence rates (LRR) to around 10% in many population studies [Citation1,Citation2] but patients with “ugly” tumours still have a substantial risk of not having R0 surgery or a local failure.
In patients with “bad” resectable rectal cancer the advantages of preoperative CRT includes reduced LRR; the potential to increase the likelihood of a R0 resection; an improved chance of sphincter preservation achieved by downsizing; less acute toxicity; enhanced radio-sensitivity because of better oxygenated cells and better compliance [Citation18].
This is in contrast to the situation in patients with true non-resectable LARC (“ugly”) in which tumour-shrinkage is absolutely necessary to make the primary tumour resectable. Standard therapy in these patients is most often preoperative CRT with a radiation dose of at least 45 Gy with concomitant 5-FU-based CT with the goal of a R0 resection [Citation19]. This strategy was confirmed in a randomised Swedish phase III study comparing preoperative long-course RT with CRT. CRT increased the chance of R0 resection, improved local control and there was also a trend towards improved overall survival (66% vs. 53%; p = 0.09) [Citation20].
In our prior phase I and subsequent phase II trial evaluating preoperative CRT (60 Gy/30 fractions with concomitant UFT) in patients with non-resectable rectal cancer we found major tumour regression in most patients, a resection rate of 77% and pCR in 13% [Citation5,Citation12]. Even though we used high-dose RT with 60 Gy the toxicity was very modest and therefore we considered and planned a more dose-intense regimen with, hopefully, an improved efficacy.
In a large review of prospective studies in patients with resectable RC, Hartley et al. found that the use of continuous infusion 5-FU, the use of a second drug (often Ox or irinotecan) and radiation dose were associated with higher rates of pCR [Citation11]. In addition, phase II studies have shown the feasibility of combining 5-FU and Ox with preoperative RT with promising pCR rates around 20% [Citation7,Citation21,Citation22].
Based on the above facts, we decided for a phase I study to investigate the feasibility of combining high-dose CRT (60 Gy with daily oral UFT) with concurrent dose-escalating weekly Ox in patients with non-resectable LARC. Systemic relapses constitute a major problem in patients with rectal cancer and therefore we added one course of UFT/Ox before CRT and one course after CRT but before planned surgery.
Patients who achieve a pCR have an improved long-term outcome in terms of LRR and OS independent of the initial clinical T and N stage [Citation15,Citation23,Citation24]. However, the promising pCR rates from phase II could not be confirmed in randomised trials [Citation25–27]. In the randomised ACCORD 12/0405 study comparing preoperative RT 45 Gy plus capecitabine with 50 Gy plus capecitabine plus Ox 50 mg/m2/week in patients with resectable T3-4 M0 RC the benefit of Ox could not be demonstrated in terms of the pCR rate (14% vs. 19%). There was no difference in the amount of patients developing distal metastases in the two groups [Citation28].
The Italian Studio Terapia Adiuvanta Retto (STAR) trial also compared preoperative RT plus capecitabine with RT plus capecitabine plus Ox in the same group of patients as in the ACCORD trial [Citation29]. Thus the RT dose was 50.4 Gy in both groups, and the Ox dose was slightly higher, 60 mg/m2/week. Early results showed no significant differences in pCR (16% in both arms), local tumour response or tumour down-staging compared with patients who received a standard fluorouracil-based CRT regimen. pCR has in these and other trials been used as a surrogate efficacy endpoint but long-term outcome for both studies are awaited since histopathology only indirectly can be informative of possible extra-pelvic micrometastasis.
The early delivery of systemic combination treatment before CRT and TME could be a more tolerable means of delivering an effective systemic dose of Ox and has been investigated in phase II trials [Citation30,Citation31]. This approach is supported by results of a randomised study comparing combination CT given as induction treatment before CRT and surgery with the same CT given as adjuvant treatment, which concluded that induction treatment had better safety and a higher dose-intensity of systemic treatment [Citation32]. Chau et al. found that 71% of the patients had a relief of pain during their four cycles of XELOX preceding CRT [Citation33]. This was confirmed in the CORGI-L study [Citation30].
Should the results from the ACCORD and STAR trials be the end of the concomitant Ox? We absolutely do not think so. There is still much to investigate especially in optimising the treatment to reduce the substantially high relapse rates still seen in RC compared to the LRR. Believing in this the on-going PETACC 6 and NSABP R-04 trials questioning the intensification of preoperative CRT and postoperative adjuvant treatment should still recruit patients. The CAO/ARO/AIO-04-study closed for recruiting patients in 2010 and the final results are awaited. This intensification will also be investigated in the RAPIDO phase III study (EudraCT number 2010-023957-12) where the short-course RT as well will be part of the investigation in patients with LARC. Conclusively, the results of our single-institution study confirmed that the addition of weekly Ox to preoperative combination treatment with high-dose RT for un-resectable LARC was feasible with an acceptable toxicity profile. Data on ypCR are encouraging in patients with huge, fixed adenocarcinomas of the rectum taking in account that high pCR rates are seen in significantly more patients with lower T-stage tumours [Citation24].
Efficacy data of the present study are very encouraging as well, with little grade 3–4 toxicity and therefore it would be natural to continue with the planned phase II part of this study. However, phase III studies add more to the overall knowledge and therefore we are planning to participate in the ongoing RAPIDO trial.
The study received a medical grant from Merck KGaA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol 2005;31:845–53.
- Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142–9.
- Glimelius B, Holm T, Blomqvist L. Chemotherapy in addition to preoperative radiotherapy in locally advanced rectal cancer – a systematic overview. Rev Recent Clin Trials 2008; 3:204–11.
- O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, .Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502–7.
- Vestermark LW, Jacobsen A, Qvortrup C, Hansen F, Bisgaad C, Baatrup G. . Long-term results of phase II trial of high-dose radiotherapy (60 Gy) and UFT/ l-leukovorin in patients with non-resectable locally advanced rectal cancer (LARC). Acta Oncol 2008;47:428–33.
- Sadahiro S, Suzuki T, Kameya T, Iwase H, Tajima T, Makuuchi H. A pharmacological study of the weekday-on/weekday-off UFT schedule in colorectal cancer patients. Cancer Chemther Pharmacol 2001;47:447–50.
- Rodel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R, Phase I/II trial of capecitabine oxaliplatin, and radiation for rectal cancer. J Clin Oncol 2003;21:3098–104.
- Aschele C, Friso ML, Pucciarelli S, Lonardi S, Sartor L, Fabris G. . A phase I-II studyof weekly oxaliplatin 5-fluorouracil continuous infusion and preoperative radiotherapy in locallyadvanced rectal cancer. Ann Oncol2005;16:1140–6.
- Mohiuddin M, Regine WF, John WJ, Hagihara PF, McGrath PC, Kenady DF, . Preoperative chemoradiation in fixed distal rectal cancer: Dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys2000;46:883–8.
- Glimelius B, Isacsson U, Jung B, Påhlman L. Radiotherapy in addition to radical surgery in rectal cancer: Evidence for a dose-response effect favouring preoperative treatment. Int J Radiat Oncol Biol Phys 1997;37:281–7.
- Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: Analysis of phase II/III trials. Br J Radiol 2005;78:934–8.
- Pfeiffer P. High-dose radiotherapy and concurrent UFT plus l-leukovorin in locally advanced rectal cancer: A phase I trial. Acta Oncol 2005;44:224–9.
- Glynne-Jones R, Meadows H, Wood W. Chemotherapy or no chemotherapy in clear margins after neoadjuvant chemoradiation in locally advanced rectal cancer: CHRONICLE. A randomised phase III trial of control vs. capecitabine plus oxaliplatin. Clin Oncol (R Coll Radiol) 2007;19:327–9.
- Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomized trials. Ann Oncol 2010;21:1743–50.
- Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M. . The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752–;60.
- Rodel C, Sauer R. Integration of novel agents into combined-modality treatment for rectal cancer patients. Strahlenther Onkol 2007;183:227–35.
- Blomqvist L, Glimelius B. The “good”, the “bad”, and the “ugly” rectal cancers. Acta Oncol 2008;47:5–8.Editorial.
- Glynne-Jones R, Grainger J, Harrison M, Ostler P, Makris A. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious?Br J Cancer 2006;94:363–71. Review.
- Valentini V, Glimelius B. Rectal cancer radiotherapy: Towards European consensus. Acta Oncol 2010;49:1206–16.
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L. . Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687–94.
- Ryan DP, Niedzwiecki D, Hollis D. Phase I-II study of preoperative oxaliplatin fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and leukaemia Group B 89901. J Clin Oncol 2006;24:2557–62.
- Rodel C, Liersch T, Hermann RM, Arnold D, Reese T, Hipp M. . Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 2007;25:110–7.
- Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R . Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99–107.
- Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, . Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, .Chemotherapy with preoperative radiotherapy in rectal cancer. New Engl J Med2006;355:1114–23.
- Gerard J-P, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, . Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006;24:4620–5.
- Bujko K, Michalski W, Kepka MP, Nowacki MP, Nasierowska-Guttmeier A, Tokar P, .Association between pathologic response in metastatic lymph nodes after preoperative chemotherapy and risk of distant metastases in rectal cancer: An analysis of outcomes in a randomized trial. Int J Radiat Oncol Phys 2007;67:369–77.
- Gerard J-P, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, . Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638–43.
- Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, . Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773–80.
- Gunnlaugsson A, Anderson H, Fernebro E, Kjellen E, Bystrom P, Berglund K, . Multicentre phase II trial of capecitabine and oxaliplatin in combination with radiotherapy for unresectable colorectal cancer: The CORGI-L study. Eur J Cancer 2009;45:807–13.
- Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, . Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: A phase II trial. Lancet Oncol 2010;11:241–8.
- Fernandez-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, . Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010;28:859–65.
- Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, . Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006;24:668–74.
