Abstract
Background. To investigate the utility of stereotactic body radiotherapy (SBRT) in the treatment of painful renal cell carcinoma (RCC) bone metastases, and for a possible dose effect on time to symptom relief. Material and methods. Eighteen patients with 24 painful osseous lesions from metastatic RCC were treated with SBRT. The most common treatment regimens were 24 Gy in 3 fractions and 40 Gy in 5 fractions. The times from treatment to first reported pain relief and time to symptom recurrence were evaluated. Median follow-up was 38 weeks (1–156 weeks). Results. Seventy-eight percent of all patients had pain relief. Patients treated with a BED > 85 Gy achieved faster and more durable pain relief compared to those treated with a BED < 85 Gy. There was decrease in time to pain relief after a change in treatment regimen to 8 Gy × 5 fractions (BED = 86). There was only one patient with grade 1 skin toxicity. No neurological or other toxicity was observed. Conclusions. SBRT can safely and effectively treat painful RCC bony metastases. There appears to be a relationship between radiation dose and time to stable pain relief.
Bone metastases are a common feature of many solid cancers, especially those originating from the prostate, breast, kidney, and lung. Up to 80% of patients with metastatic disease will develop painful bony disease during the course of their disease.
Both conventional radiotherapy and surgery have a described role in the acute treatment of bony metastatic disease. Surgery has historically been used in cases with bone/spinal instability, history of previous radiotherapy, or in cases of perceived radioresistant tumor types. Conventional radiotherapy is effective in the palliation of symptoms, such as pain and neurological dysfunction, in the majority of patients with various tumor types [Citation1–3].
Stereotactic body radiotherapy (SBRT), using higher fraction size in few fractions, is effective in the treatment of renal cell carcinoma (RCC) spinal metastases [Citation4]. This retrospective paper examines the use of SBRT in the palliation of painful RCC metastases in various bony sites, with a primary endpoint being time to stable resolution of pain, which has been hypothesized to be quicker when compared to conventional radiotherapy. Specifically, we have examined the effect of SBRT on RCC bone metastases, a pathology that has traditionally been labeled as radioresistant when treated with conventional external beam radiotherapy [Citation5].
Material and methods
From April 2004 to March 2006, 18 patients with 24 osseous lesions () were treated with SBRT using the BrainLAB Novalis stereotactic system. Immobilization was utilized with the vacuum body cast system (BodyFix, Medical Intelligence, USA) and daily image guidance was done prior to each treatment with kV x-rays. ExacTrac and 6-D robotic couch were utilized.
Table I. Patient characteristics and outcomes divided into those treated with BED < 85, BED > 85 and all lesions.
Patient selection
All patients had pathology proven RCC, with magnetic resonance imaging (MRI) or computed tomography (CT) documentation of bony metastatic disease. None of the patients had evidence of spinal cord compression or previous radiation to the tumor site. Additionally, all patients were evaluated by either a neurosurgeon or orthopedic surgeon for spinal instability and fracture risk. Patients who were deemed to be at fracture risk were not treated with SBRT. They either received surgical intervention followed by conventional radiotherapy doses or conventional radiotherapy alone.
All histologies were clear cell RCC. All tumors were smaller than 7 cm. Tumors were located in the spine, ribs, clavicles, and pelvis (). More specifically, 14 lesions were located in the vertebral column; four in the ribs; and six in the pelvis. The vertebral lesions were at least 5 mm from the spinal cord to allow for adequate dose fall off. Proximity to other critical normal tissues, for example the small bowel, was also taken into consideration.
Full planning and treatment process with the Novalis system has been described by our group previously [Citation6].
Delineation of the GTV and PTV
Gross tumor volume (GTV) was defined as the area of disease seen on CT-scan and MRI. In addition, if the patient had a positron emission tomography (PET)/CT-scan done, the PET images were fused with the simulation CT and PET avid areas contributed to GTV delineation. To allow for daily set up error, planning target volume (PTV) was created by adding 5 mm to the GTV, with the exception of margin extension into critical normal structures, such as the spinal cord and bowel.
To account for respiratory motion, all patients with rib and clavicle lesions had a 4-D CT simulation while immobilized in a vacuum molded body cast (Medical Intelligence). An internal target volume (ITV) was created by delineating the GTV on a maximal intensity projection (MIP) data set.
Dose delivery and constraints
SBRT treatment planning and delivery was done with intensity modulated radiation therapy (IMRT) or dynamic conformal arcs. The goal was to create a very sharp dose fall off to spare critical non-malignant tissue (e.g. spinal cord) and deliver a focused ablative radiation dose to the tumor. Prior to each SBRT treatment, ExacTRAC stereoscopic KV x-rays were taken for accurate image guidance.
For target dose planning and delivery, 100% of the PTV had to be covered by at least 90% of the prescribed dose. This was feasible in all patients due to patient selection for SBRT with regards to tumor distance from at risk organs. Dose was prescribed to 90–100% isodose volumes, depending on the size and location of the target tumor. Target dose heterogeneity was allowed with up to 110% of prescription dose allowed within the target volume. For spinal lesions, max dose to the spinal cord did not exceed 4 Gy per fraction. Total dose to 10% of the near field spinal cord volume did not exceed 20 Gy. Near field cord was defined as the cord in the same axial slices as the target, plus cord extending 6 mm inferior and 6 mm superior to the target.
Dose volume histogram (DVH) of the targets and normal tissues were evaluated for all patients.
Dose and fractionation regimens
Multiple dosing regimens were used in the treatment of the bony metastases; the most common regimens were 24 Gy in 3 fractions (n = 8) and 40 Gy in 5 fractions (n = 9) (). Because of these non-uniform dosing regimens, a biological equivalent dose (BED) was calculated for all patients treated by using the following formula ():
Table II. Number of lesions treated and various dose/fractionation schemes utilized.
BED = (TD) [1 + d/(α/β)]
Where TD = total dose, d = dose per fraction, and α/β = 7. Previous studies have utilized an α/β of 3 and of 7 for RCC given the relatively radioresistant nature of RCC, as well as the more conventional α/β of 10 for general tumor kill [Citation7,Citation8]. We chose seven as our primary α/β value for the purpose of comparison between dosing regimens.
Analysis
A retrospective analysis was done on these patients evaluating the relief of acute pain secondary to tumor, and time to recurrence. Median follow-up of this cohort was 38 weeks (range 1–156 weeks).
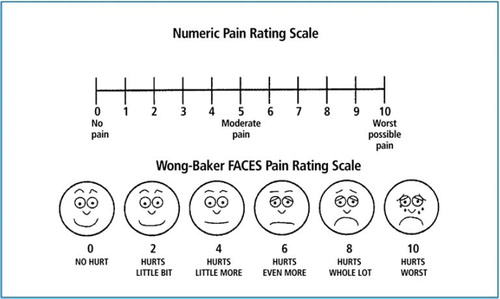
Pain was rated on a 1–10 scale by the patients on a modified visual and verbal Wong-Baker Faces Pain Rating scale upon initial presentation, then again at the end of treatment, and then at each subsequent follow-up (). Pain relief was defined as a patient reported decrease in pain score at follow-up visits. Pain recurrence was any increase in patient reported pain relief score. Treatment related toxicity was also assessed with the NCI-CTC grading system.
Results
Pain response
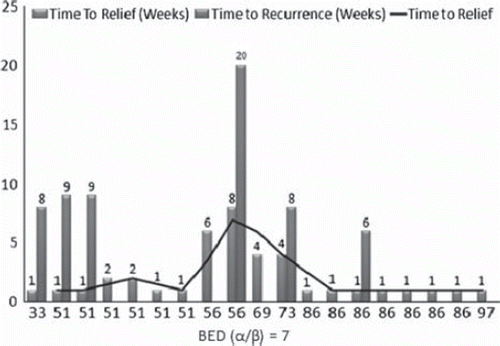
One hundred percent of patients had pain reported at the lesion site prior to treatment. At a mean follow-up of 38 weeks, 78% of patients had symptomatic relief (). Of all patients, the mean time to first pain relief was approximately two weeks. Thirty-two percent of those had a symptomatic recurrence at a mean of 10 weeks.
Of note, there was a decrease in time to patient relief after a change in treatment regimen to 8 Gy × 5 fractions. As shown in , in those patients treated with a BED > 85, mean time to decrease in pain score was one week, and 83% of patients had a response. Only one patient in the high dose group had a symptom recurrence which was at six weeks.
All pain evaluation and reporting was recorded independent of pain medication changes or reduction. Of the 14 patients who achieved some pain relief, three reported complete reduction of opiate medication. The remainder had stable or mild decrease in pro re nata medication use. There was no observable relationship between dosing regimen and pain medication changes.
Dose response
In the analysis of time to symptom relief and BED administered, there appeared to be a dose response trend (). For an α/β of 7, in patients treated to a BED greater than 85 Gy, pain relief was more consistent and quicker, as compared to patients treated with BED less than 85 Gy. Additionally, though the sample size is small, there appeared to be a trend for a lower number of painful recurrences in those treated with greater than 85 Gy.
Figure 2. Nineteen of 24 lesions responded to treatment. X-axis is BED; Y-axis is weeks. Each blue bar indicates the time to first relief of a single patient; each red bars indicates the time to recurrence, if applicable. Black line is a moving average of time to symptomatic response across all responsive patients.
Toxicity
There was one patient with grade 1 skin toxicity. No neurological or other toxicity was observed.
Discussion
In general, SBRT may offer a quicker resolution of symptoms when compared to traditional radiotherapy due to the ability to limit dose to critical structures while enabling hypofractionation with higher fraction size to the disease. There have been various studies showing that with conventional radiation, pain levels stabilize anywhere from four to six weeks to three months after treatment in a variety of tumors that have metastasized to the bone [Citation9,Citation10]. This retrospective study showed that with SBRT the average pain stabilization time was two weeks (one week in the higher dose SBRT group) in the setting of RCC metastases, which are considered to be radioresistant when treated with conventional fractionation.
The unique role, advantages, and disadvantages associated with the use of SBRT in RCC and non-RCC bone metastases have been discussed in the literature previously by our group [Citation11]. Promising clinical results have been shown with the use of SBRT in the setting of primary and metastatic RCC [Citation4,Citation5,Citation12–14,Citation18].
To the best of our knowledge, there are no randomized trials evaluating SBRT hypofractionation versus single fractionation dosing regimens as there are in the conventional radiotherapy literature [Citation3]. Various reports have been published by other institutions that have varied dosing regimens from 6–30 Gy in 1–5 fractions [Citation15,Citation16,Citation18].
It has been hypothesized that these higher doses will result in better local control rates and longer duration of symptom alleviation [Citation17]. This is especially true for RCC, a pathology considered radioresistant when treated with conventional dosing [Citation18]. It is for this reason that we analyzed the relationship between dosing regimen and time to resolution of pain. Based on the data presented in this study, treating to a BED ≥ 85 Gy (α/β of 7) resulted in a trend towards quicker resolution of pain (). In this higher dose group, the most common regimen utilized was 8 Gy × 5 fractions (). This is the most consistently effective regimen in this study, although the number of patients is small. We are investigating this regimen in a prospective trial.
Previously published data confirms that ablative or near ablative dose per fraction delivery with SBRT can allow for successful symptomatic management in radioresistant RCC [Citation4,Citation5,Citation18,Citation19]. In a 2005 study by the University of Pittsburgh neurosurgery group, Gerszten et al., reported on 60 RCC spinal lesions, 42 of which had previously failed on conventional external beam radiotherapy, treated with SBRT in a single mean fraction of 20 Gy. Eighty-nine percent of the patients experienced an improvement in their pain after SBRT, and seven of eight patients who were treated for tumor progression had documented disease control [Citation18]. The authors concluded that when external beam radiotherapy offers poor control of RCC, spinal radiosurgery may offer a viable solution [Citation18]. Huguenin et al. reported on a prospective trial which utilized multiple radiation dosing regimens in the treatment of patients with metastatic melanoma and RCC. Patients received a variety of regimens ranging from 30 Gy in 10 fractions, 20 Gy in 10 fractions, 20 Gy in 5 fractions, or 24 Gy in 3 fractions. They reported a 63% reduction in pain that lasted for more than four weeks [Citation19].
Most recently, Nguyen et al. showed that SBRT in the setting of RCC spinal metastases can be effectively treated with hypofractionation. In their investigation, 48 patients were treated with either 24 Gy in a 1 fraction, 27 Gy in 3 fractions, or 30 Gy in 5 fractions. Fifty-eight percent of their patients had previous radiation. At pretreatment baseline, 23% patients were pain free; at one month and 12 months post SBRT, 44% and 52% patients were pain free, respectively. No Grade 3–4 neurologic toxicity was observed [Citation4].
These results differ from the data presented here. Additionally, two important differences in patient characteristics should be pointed out. The current study analyzes patients with no previous radiation at the sites of disease which allowed our dosing regimen to deliver a higher BED in our high-dose group of patients. Additionally, the current study analyzes the use of SBRT for various anatomical bony sites and is not only limited to the spine. This also allows a higher BED to be delivered when cord tolerance is not an issue.
In terms of recurrence, our study found that after a median follow-up of about 38 weeks, 32% of cases experienced a symptomatic recurrence, with 12% in the high BED group, and 46% in the lower BED group. Ryu et al. retrospectively reported on 49 patients with spinal metastases of various pathologies who were treated with a single fraction of stereotactic radiosurgery from 10–16 Gy. Their recurrence rate at the treated site was 7% during postradiotherapy follow-up [Citation20]. Our overall recurrence rate is higher, possibly due to the fact that all the patients in this study had RCC. In the higher BED group, the recurrence rate was similar.
Many of the inherent weaknesses in this study can be attributed to the fact that this is a retrospective report on initial experiences. Because the dosing regimens were mixed, beyond noting a general trend, it is hard to draw very specific conclusions from the results of this study. By converting all dosing regimens to BED, some degree of standardization was introduced for analysis of the dosing regimens. Additionally, all patients had RCC. An upcoming prospective trial will help resolve these issues and further clarify the trends seen here.
Conclusions
Initial experiences suggest that SBRT can be used to treat patients with painful RCC bony metastases in a variety of anatomic locations safely and effectively. The data also suggests a dose-response with regards to time to symptom resolution, with a distinction seen at a BED of > 85 Gy with an α/β of 7. The most consistently effective dosing regimen used was 40 Gy given in 5 fractions.
Acknowledgements
This research was partly presented at the American Radium Society Annual Meeting, Cancun, Mexico, May 1–5, 2010. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- McQuay HJ, Collins S, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev 2000;2.
- Steenland E. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101–9.
- Sze WM, Shelly MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: Single fraction versus multi-fraction radiotherapy: A systemic review of randomized trials. Clin Oncol 2003;15:345–52.
- Nguyen QN, Shiu AS, Rhines LD, Wang H, Allen PK, Wang XS, . Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1185–92.
- Svedman C, Sandstrom P, Pisa P, Blomgren H, Lax I, Kälkner KM, . A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006;45:870–5.
- Teh BS, Paulino AC, Lu HH, Chiu JK, Richardson S, Chiang S, . Versatility of the novalis system to deliver image-guided stereotactic body radiation therapy (SBRT) for various anatomical sites. Technol Cancer Res Treat 2007; 6:347–54.
- Nelson JW, Yoo DS, Sampson JH, Isaacs RE, Larrier NA, Marks LB, . Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys 2009;73:1369–75.
- Wilson D, Hiller L, Gray L, Grainger M, Stirling A, James N. The effect of biological effective dose on time to symptom progression in metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol) 2003;15:400–7.
- Agarawal J, Swangsilpa T, van der Linden Y, Rades D, Jeremic B, Hoskin PJ. The role of external beam radiotherapy in the management of bone metastases. Clin Oncol 2006;18:747–60.
- Greenberg H, Kim J, Posner J. Epidural spinal cord compression from metastatic tumor: Results of a new treatment protocol. Ann Neurol 1980;8:361–6.
- Jhaveri P, Teh BS, Bloch C, Amato R, Butler EB, Paulino AC. Stereotactic body radiotherapy in the management of painful bone metastases. Oncology (Williston Park) 2008;22:782, 8; discussion 788–9, 796–7.
- Teh BS, Bloch C, Paulino AC, Shen S, Hinckley L, Baskin D, . Pathologic complete response in renal cell carcinoma brain metastases treated with stereotactic radiosurgery. Clin Genitourin Cancer 2007;5:334–7.
- Doh L, Curtis AE, Teh BS. Renal-cell carcinoma. N Engl J Med 2006;354:1095,6; author reply 1095–6.
- Teh BS, Bloch C, Galli-Guevara M, Doh L, Richardson S, Chiang S, . The treatment of primary and metastatic renal cell carcinoma (RCC) with image-guided stereotactic body radiation therapy (SBRT). Biomed Imaging Interv J 2007;3:e6.
- De Salles AA, Pedroso AG, Medin P, Agazaryan N, Solberg T, Cabatan-Awang C, . Spinal lesions treated with Novalis shaped beam intensity-modulated radiosurgery and stereotactic radiotherapy. J Neurosurg 2004;101:435–40.
- Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine 2007;32:193–9.
- Milker-Zabel S, Zabel A, Thilmann C, Schlegel W, Wannenmacher M, Debus J. Clinical results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003;55:162–7.
- Gerszten P, Burton S, Ozhasoglu C, Vogel WJ, Welch WC, Baar J, . Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine 2005;3:288–95.
- Huguenin PU, Kieser S, Glanzmann C, Capaul R, Lütolf UM. Radiotherapy for metastatic carcinomas of the kidney or melanomas: An analysis using palliative end points. Int J Radiat Oncol Biol Phys 1998;41:401–5.
- Ryu S, Rock J, Rosenblum M, Kim JH. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg 2004;101:402–5.