To the Editor,
Colorectal cancer has among the highest incidence and mortality rates in the Western world, with rates continuing to rise [Citation1]. With five-year survival for stage I and II disease of approximately 90% and 80%, respectively, and significantly lower survival in more advanced disease [Citation2], early diagnosis is an essential element to decreasing colorectal cancer mortality. However, in the early stages the disease is asymptomatic thereby highlighting the importance of screening for increased early detection. Current screening method, such as fecal occult blood testing and colonoscopy are limited in that they lack specificity or are expensive and invasive, respectively. This, along with increases in colorectal cancer worldwide incidence and rising healthcare costs, highlight the need for a minimally invasive, inexpensive screening test; with assays detecting specific serum or blood-based markers representing an ideal diagnostic method. Identification of novel blood-based markers able to detect colorectal cancer is therefore important, with autoantibodies to colorectal cancer specific tumour associated antigens representing a novel source of such markers. Using protein macrorarrays, we have previously identified a panel of autoantibody markers present in the sera of colorectal cancer patients [Citation3], including autoantibodies to TRIM28, whose main function as a transcriptional repressor; due in part to its role in epigenetic modifications [Citation4,Citation5]. TRIM28 expression has also been shown to be elevated in cancer [Citation6,Citation7]. Here, using TRIM28 as the tumour associated antigen, we determined the suitability of reverse ELISAs (enzyme-linked immunosorbent assay) for the detection of colorectal cancer specific antibodies originally identified using protein microarrays.
Serum samples from colorectal cancer patients (n = 58) and healthy controls (n = 55) were collected from patients undergoing colonoscopy at Beaumont Hospital, Dublin, Ireland and stored at -80°C. Ethical approval was obtained for this study by the Beaumont Hospital Ethics (Medical Research) Committee, with informed consent obtained from all patients. A diagnosis of colorectal cancer was confirmed by a pathologist, and patients with a history of cancer, autoimmune disease or systemic inflammatory conditions or taking immunosuppressive agents were excluded from the study. Patients in the control arm of the study were drawn from those patients with normal colonoscopies. Clinical characteristics of the colorectal cancer and non-cancer control arm of the study are indicated in .
Table I. Details of patient cohort.
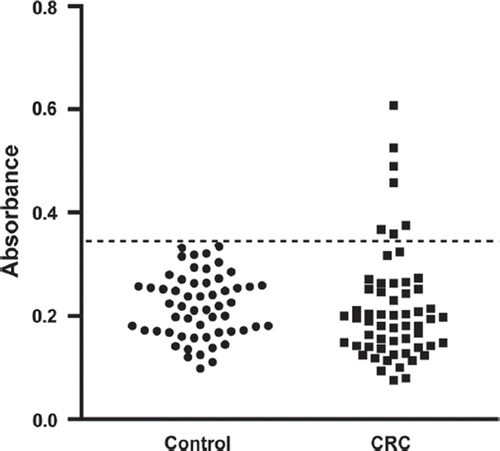
TRIM28 recombinant protein was expressed in Escherichia coli clones from the hEx1 library and purified using FPLC, with purity and size verified using SDS-PAGE and Commassie Blue staining. Serums from colorectal cancer patients (n = 58) and healthy controls (n = 55) were diluted (1:50) and were screened using a reverse ELISA optimised for the detection of TRIM28 autoantibodies (). Positivity for TRIM28 autoantibodies for an individual patient was defined as an absorbance value greater than two standard deviations above the mean absorbance of non-cancer control sera. Using this threshold, autoantibodies to TRIM28 were present in a significant number of colorectal cancer patient serums compared to non-cancerous controls, being detected in the serums of seven colorectal cancer patients and no non-cancerous controls (Fisher's exact test; p = 0.013), corresponding to a sensitivity of 12.1% and a specificity of 100%. Importantly, antibodies to TRIM28 were detected in 18.2% of early stage I and II disease (4/22) compared to 8.3% of patients with more advanced stage III and IV disease (3/36).
Figure 1. Detection of antibodies to TRIM28 in colorectal cancer (‘CRC’; n = 58) and control (n = 55) patient serum as determined by reverse ELISA conditions. Seropositivity in colorectal cancer patients was determined by whether an individual's absorbance value was greater than control mean absorbance + 2SD (dashed line).
Discussion
The mechanisms by which tumour associated antigens develop are not well understood, but may be attributable to alterations in protein expression resulting from genetic mutations and/or altered protein processing in tumour cells, ultimately resulting in an immune response and autoantibody production [Citation8]. Serum autoantibodies have been detected in early stage cancers [Citation9,Citation10] and therefore have significant potential as cancer diagnostic markers. Using a cohort of colorectal cancer patients and non-cancerous controls, we demonstrate here that autoantibodies to TRIM28 were detectable in a significant number of colorectal cancer patient serums compared to controls. Importantly we also demonstrate that seropositivity to TRIM28 autoantibodies was not limited to a specific disease stage, but rather patients of all disease stages showed some positivity, further highlighting the potential of TRIM28 in detecting early stage disease. This work represents a proof-of-concept study demonstrating the ability of reverse ELISA assays to detect serum autoanibodies originally detected using protein macroarrays.
The ideal diagnostic test will have both high specificity and sensitivity. While this study indicates that TRIM28 serum autoantibodies were highly specific for colorectal cancer; there was little sensitivity with antibodies detected in only seven cancer patients. However, this does not preclude the diagnostic potential of TRIM28 autoantibodies. One recent study aimed at the detecting a number of different serum autoantibodies in colorectal cancer demonstrated that individual autoantibodies often demonstrated low sensitivity and high specificity for colorectal cancer detection, however when different autoantibodies were taken together, sensitivity increased to 58.5% without great sacrifice to specificity (92.6%) [Citation11]. TRIM28 was first identified as part of a larger panel of autoantibodies specifically present in the serum of colorectal cancer patients. Studies aimed at the detection of these autoantibodies in combination with TRIM28 will likely result in a panel of markers with vastly improved sensitivity. Inclusion of other blood markers, such as autoantibodies to p53 and CEA levels may also serve to increase the diagnostic sensitivity without sacrificing specificity [Citation11,Citation12].
Importantly, identification of tumour associated antigens may provide insight into the molecular events occurring during carcinogenesis. Importantly TRIM28 expression has also been associated with and metastasis and poor prognosis in breast [Citation6] and gastric [Citation7] cancers. TRIM28 was first identified as a transcriptional co-repressor in KRAB mediated transcriptional repression [Citation13] but has also been implicated in epigenetic gene silencing [Citation4] through its interactions with the histone methyltransferase SETDB1 [Citation5] and histone deacetylase [Citation14,Citation15]. Other TRIM28 functions include involvement in DNA repair [Citation16], mdm2 mediated degradation of p53 [Citation17] and the epithelial-to-mesenchymal transition [Citation18]. These molecular functions, along with its altered expression in cancers indicate that TRIM28 may play a role in both the development of malignancy as well as chemotherapy response. While the study presented here examine associations between the presence of serum autoantibodies and clinical outcome, further studies examining this as well as any differential expression of TRIM28 in colorectal tumours and further mechanistic molecular are warranted.
In conclusion, this study demonstrates the suitability of reverse ELISAs for the detection of autoantibodies originally identified using protein arrays. In addition, we demonstrate the high level of specificity of TRIM28 autoantibodies for colorectal cancer. Addition of other blood-based tumour markers in a larger cohort will give additional insight into their ability to detect colorectal cancer in early stage disease, ultimately aiding in the development of minimally invasive diagnostics and more effective, less costly screening programs.
Acknowledgements
We would like to acknowledge the work of the clinical research nurses Joan Kehoe and Deirdre Hyland for their work in the collection of patient samples and clinical data. This work was generously supported by grants from Enterprise Ireland (PC/2008/0366) to SH, the Health Research Board (HRA_POR/ 2010/116) to JHMP and the Irish Cancer Society (CRF10KIJ) to GK. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A cancer journal for clinicians 2011;61:69–90.
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420–5.
- Kijanka G, Hector S, Kay EW, Murray F, Cummins R, Murphy D, . Human igg antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut 2010;59:69–78.
- Rowe HM, Jakobsson J, Mesnard D, Rougemont J, Reynard S, Aktas T, . Kap1 controls endogenous retroviruses in embryonic stem cells. Nature 2010;463:237–40.
- Schultz DC, Ayyanathan K, Negorev D, Maul GG, Rauscher FJ, 3rd. Setdb1: A novel kap-1-associated histone h3, lysine 9-specific methyltransferase that contributes to hp1-mediated silencing of euchromatic genes by krab zinc-finger proteins. Genes Dev 2002;16:919–32.
- Ho J, Kong JW, Choong LY, Loh MC, Toy W, Chong PK, . Novel breast cancer metastasis-associated proteins. J Proteome Res 2009;8:583–94.
- Yokoe T, Toiyama Y, Okugawa Y, Tanaka K, Ohi M, Inoue Y, et al. Kap1 is associated with peritoneal carcinomatosis in gastric cancer. Ann Surg Oncol 2010;17:821–8
- Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest 2001;108:1411–5.
- Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer her-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 1997;15:3363–7.
- Tomaino B, Cappello P, Capello M, Fredolini C, Ponzetto A, Novarino A, . Autoantibody signature in human ductal pancreatic adenocarcinoma. J Proteome Res 2007;6:4025–31.
- Chan CC, Fan CW, Kuo YB, Chen YH, Chang PY, Chen KT, . Multiple serological biomarkers for colorectal cancer detection. Int J Cancer 2010;126:1683–90.
- Muller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, . Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol 2006;29:973–80.
- Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, . Kap-1, a novel corepressor for the highly conserved krab repression domain. Genes Develop 1996;10:2067–78.
- Jakobsson J, Cordero MI, Bisaz R, Groner AC, Busskamp V, Bensadoun J-C, . Kap1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron 2008;60:818–31.
- Wolf D, Goff SP. Trim28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 2007;131:46–57.
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, . Atm signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 2008;31:167–77.
- Wang C, Ivanov A, Chen L, Fredericks WJ, Seto E, Rauscher FJ, 3rd, . Mdm2 interaction with nuclear corepressor kap1 contributes to p53 inactivation. EMBO J 2005;24:3279–90.
- Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, . A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest 2007;117:482–91.
