Abstract
Background. Interest in boosting the dose to the tumour during neo-adjuvant radiochemotherapy for rectal cancer is ever increasing, especially within the frame of adaptive radiotherapy. Rectal motion remains a potentially important obstacle to the full exploitation of this approach and needs to be carefully investigated. Material and methods. The main purposes of this work were to: a) quantify rectal motion on all fractions of a treatment course; and b) assess margins for adaptive boosting in the second part of the treatment in order to benefit of tumour reduction during treatment. Ten consecutive patients treated with image-guided tomotherapy (41.4 Gy, 18 fractions) were selected. The cranial half of the rectum (subject to motion) was contoured by a single observer on daily MVCTs. The variations of rectal volume and of the envelope of rectum positions were investigated (169 MVCTs). The impact of applying different margins to the rectum in including all its possible positions was also investigated when considering the planning kVCT, the first fraction MVCT, the half-treatment MVCT or the median rectal contours of the whole or second half of treatment as reference volumes. Results. Rectal volume reduced during treatment in all patients, with a significant time-trend in 6/10 patients. The median values of the envelope volumes were 129 cm3 and 87 cm3 in the first and second half of the treatment, respectively. On average, 95% of the rectal envelope was included by an isotropic expansion of 12 mm and 5 mm of the median contours when considering the whole or the second half of the treatment, respectively. Conclusion. A significant reduction of rectal volume was found in the second part of the treatment where rectal mobility was limited. As a consequence, relatively small margins may be used around the residual tumour volume when adaptive boost is delivered in the second half of the treatment.
Neoadjuvant treatment of stage T3-T4/anyN, operable rectal adenocarcinoma has a beneficial impact on loco-regional control, improves the pathological complete response rate and is considered as one of the most popular therapeutic options [Citation1–4].
The achievement of a high pathological complete remission (pCR) rate could be of great importance as it is strongly associated with good prognoses [Citation4]; as a consequence, the pCR rate is currently considered the primary end point of many phase II trials.
The intensification of the chemotherapeutic and/or the radiotherapy parts increased the number of pCRs to 25–30% in the best series [Citation1,Citation4,Citation5]. Focusing on the radiotherapy part, biological dose escalation was often carried out through hyper-fractionation [Citation6], while IMRT reduced toxicity in conventional and moderate hypofractionation schedules [Citation7–9]. On the other hand, proper guidelines for CTV delineation [Citation10] and the availability of image-guided techniques [Citation11] reduce the risk of geographical miss.
Recent studies have focused on the impact of mesorectum motion/deformation during a treatment course [Citation11–14], while little information is available concerning rectal (and GTV) motion [Citation15,Citation16].
The large availability of IGRT and of advanced delivery modalities is also opening new possibilities for the exploration of “adaptive radiotherapy” (ART) strategies; as most rectal cancers are known to shrink significantly during treatment, the possibility of escalating the dose to the residual GTV is attractive in order to increase the number of pCRs and/or reduce surgery side effects or improve sphincter preservation [Citation8,Citation17]. Moreover, it is known that a number of patients could potentially avoid surgery after radiochemotherapy [Citation18]; thus, the possibility to significantly escalate the dose to the residual GTV could, in principle, contribute to the exclusion of surgery for a greater proportion of patients. In these scenarios, the assessment of rectal/GTV mobility is of paramount importance.
Before introducing a pilot ART study, an investigation into rectum mobility was carried out with our moderately hypofractionated scheme [Citation9] and the major results here reported.
In order to exploit the shrinkage of GTV during treatment, our idea was to concentrate the adaptive boost to the residual tumour in the second part of the treatment, after drawing it on mid-therapy computed tomography (CT)/magnetic resonance imaging (MRI) images: the delivery would be efficiently carried out through a concomitant boost with tomotherapy.
The shrinkage of GTV would reduce the boosted volume receiving the highest dose, with a consequent reduction of any potential additional toxicity compared to the conventional, un-boosted, approach.
Based on this idea of adaptive strategy, the main purposes of the current work were: a) to quantify rectal motion on all fractions of a treatment course; and b) to derive initial estimates of margins referring to the second part of the treatment for the adaptive boost volume.
Material and methods
The clinical protocol
A phase I–II study testing moderate hypofractionated tomotherapy combined with chemotherapy in the neoadjuvant treatment of rectal cancer was activated at our institute in 2006 [Citation9]. Eligibility criteria included: histologically proven adenocarcinoma, clinical stage T3–T4 or T2 with N positive (assessed by both MR and EUS), age 18–75 years, ECOG PS ≤ 2, adequate bone marrow, renal and hepatic function. The protocol was approved by the internal review board and written informed consent was obtained from all patients. A planning contrast-enhanced CT-scan of the pelvis was performed with patients immobilised in supine position. Patients were asked to have their bladder comfortably full; no other instructions were given concerning rectum and bowel filling. CTV included tumour, mesorectum, lymph-nodes of obturator, internal iliac, common iliac chains as well as the whole anterior surface of sacrum, coccyx and piriformis muscle. Planning target volume (PTV) was defined as CTV expanded by 0.5 cm in all directions. Chemotherapy consisted of oxaliplatin 100 mg/m2 delivered on Day -14, 0, +14, and 5-FU 200 mg/m2/day from Day -14 to the end of RT (radiotherapy started on Day 0); the prescribed dose was 41.4 Gy in 18 fractions (2.3 Gy/day) and was delivered with tomotherapy. The main characteristics of the patients included in current study are summarised in .
Table I. Patient characteristics.
Tomotherapy planning and image-guidance
Field width, pitch and modulation factor were 2.5, 0.287 and 2.5, respectively. For the planning optimisation, PTV coverage and dose homogeneity had the highest priority: the fraction of PTV receiving 95% of the prescribed dose (V95%) was set ≥ 95% and maximum PTV dose (Dmax) <105%. Dmax of femoral heads was set < 42 Gy. Bladder and bowel were spared “as much as possible” in the portion outside PTV, similarly to our avoidance approach for prostate cancer [Citation19]. The dose to external genitalia was minimised by blocking any beam passage through them. Before delivering the treatment, a Megavoltage (MV) CT-scan was performed: first, an automatic bone match between planning kVCT and MVCT was carried out; then, the physician applied fine manual adjustments, if necessary, in order to minimise the residual error after bone matching due to rotations and/or deformations occurring during therapy. Daily MVCTs of 10 consecutive patients were collected and exported to the Eclipse Varian planning system.
Matching and contouring
All MVCTs images were matched with the planning kVCT using the bony anatomy as reference: the mutual information algorithm implemented in the system was used with visual verification of the goodness of the match. The choice to avoid any other “soft-tissue” match was due to the fact that the aim of the study was the estimate of rectal motion (i.e. taking bones as reference). Rectal contours were drawn by a single observer (EM) on each MVCT and then visualised on the planning kVCT. The cranial limit of the rectum was the slice where the rectum turns into the sigmoid, previously found to be robust from the point of view of contouring uncertainty [Citation20]. For the same motivation, the caudal limit was arbitrarily set at the level of the middle of the pubic simphysis, roughly splitting the rectum into two halves.
This choice was based on the following motivations: a) below this level the rectum and the anal canal are known to be stable [Citation21,Citation22]; and b) the caudal portion of the rectum is difficult to see on CT images for most patients [Citation20], especially on MVCT: thus, contouring this portion of the rectum would only increase contouring uncertainty.
Assessing volume variations
For each patient the rectal volume of each fraction as well as the union volume of all MVCT rectum contours was calculated. Then, in accordance with the main purposes of the study, the volume variation between the first and the second part of the treatment was considered separately. Rectum movement was also assessed by the DICE similarity coefficient [Citation23]: given two volumes A and B referring to two different days, the DICE index is defined as: DSC = 2. (A∩B)/(A + B).
For each patient, the average DSC of fraction k was defined as the mean DSC value when considering the agreement between the rectum at fraction k and the rectum referring to the other N-1 fractions, where N is the total number of available fractions [Citation24]. The same analysis was performed considering the average DSC values referring to different portions of the therapy.
The closer DSC is to 1, the higher the agreement is between the two contours.
A software module calculating the statistics of DSC was developed within the VODCA research software (v. 4.4.0) [Citation25].
Differences between volumes and DSC were tested by non-parametric Wilcoxon test and t-test. The correlation between rectal volume and time was tested by the Spearman test for each patient and for the whole population. The SPSS software (v. 17) was used for statistical analyses.
Estimating margins for rectal motion
In order to “safely” quantify margins to take rectal motion into account (i.e. with on-line correction of bony set-up error), we first derived the median rectal contour for each patient (corresponding to a 50% probability of including the rectum), based on the coverage probability method [Citation26]. A software module was developed in the VODCA system to carry out this analysis: the rectal volume corresponding to a certain probability of covering all the rectum positions can be calculated based on the number of contours including each voxel, creating a probability map of the possible rectum positions. Thus, the voxels included by N/2 contours (N: number of MVCTs available) defined the median contour. The median contour of both the whole treatment and the second half of the treatment, as well as the contours referring to the first fraction and to the ninth fraction (therapy midpoint), were considered as “reference” contours.
Despite the different imaging modality, the planning kVCT contour was considered as another reference (planning) contour: the treatment started generally one to two week(s) after the planning kVCT scan.
Taking the first MVCT/planning kVCTs as a reference helps in enhancing the systematic difference between the rectum before/at the start of therapy and during therapy. Using the median contour is a way of avoiding the impact of choosing one specific fraction as reference and subsequently dealing only with random uncertainties. In accordance with the main purposes of the study, the second part of the treatment was considered separately.
Thus, for each of the five reference contours, isotropically expanded reference contours were created using margins of 5 mm, 7 mm, 10 mm, 15 mm and 20 mm. The percentage fraction of the rectal union volume (envelope VE) included in the expanded reference contour (VIN) was calculated for the whole treatment and separately for the first and second half of the therapy.
Once 100 × VIN/VE is plotted against the margin, the value corresponding to large 100 × VIN/VE (for instance 95%) may be considered as a conservative estimate of the margin necessary to take rectal motion into account.
At this stage, for the sake of simplicity, only isotropic margins were considered.
Results
Volume variations and time-trend analyses
An average volume reduction of 57% was found between the start and the end of treatment; almost all reduction was observed in the first half of the treatment (average reduction: 51%).
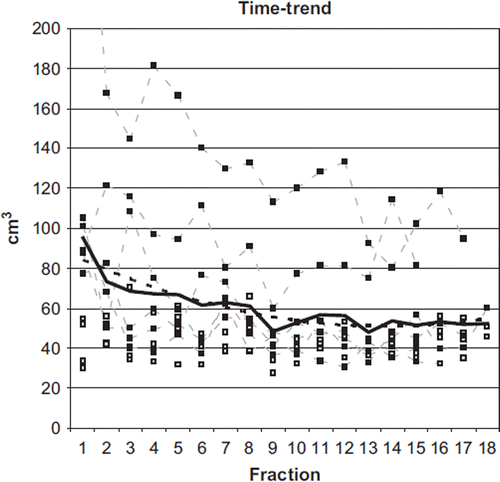
The time-trend analysis revealed a significant correlation (p ≤ 0.05) between volume and time in 6/10 patients, as shown in .
Figure 1. Time-trend analysis of rectal volume variation during treatment. Thin dotted lines (& black squares): patients with significant trend (p < 0.05); grey squares: patients without trend; continuous thick black line: average values; thick black dotted line: polynomial fit of the average trend.
On average, the Spearman's rank correlation coefficient R was −0.84 (p = 0.0005, 95% confidence interval (CI) −0.62; −0.94); of interest, R was correlated with the rectal volume at the first MVCT (p = 0.03).
The time-trend was significant in the first part of the treatment (R = −0.98, 95% CI −0.92; −0.997, p = 0.005) but not when considering the second part (R = −0.42, 95% CI −0.85; 0.34 p = 0.24), confirming that the rectal volume reduction occurred in the first fractions.
By fitting the data with a polynomial curve, as shown in , the average reduction was found to be around 35 cm3 in the first 9 fractions (4 cm3/fraction). When considering the envelope volumes, the values referring to the first part of the treatment were always larger than those referring to the second half () with average values equal to 129 ± 76 cm3 and 87 ± 23 cm3 (p = 0.002), respectively.
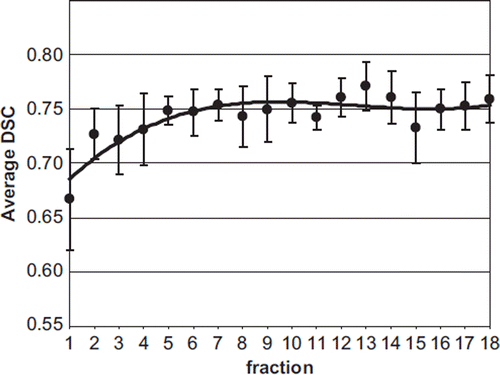
Contour agreement analysis using DSC
Mean DSC values were found to increase after the first few fractions (). The mean DSC referring to the first fraction was significantly worse than the mean DSC value referring to all the other fractions (0.67 ± 0.09 vs. 0.75 ± 0.03; p < 0.00001, t-test). Similarly, the average value referring to the first four fractions was worse than the remaining fractions (0.70 ± 0.04 vs. 0.75 ± 0.03; p < 0.00001, t-test).
Margins for rectal motion
A summary of the results is shown in and .
Figure 4. The percentage of rectal envelope (with range) included in the expanded median rectum vs. isotropic margin referring to the whole treatment (dotted) and to the second half of the treatment (continuous).
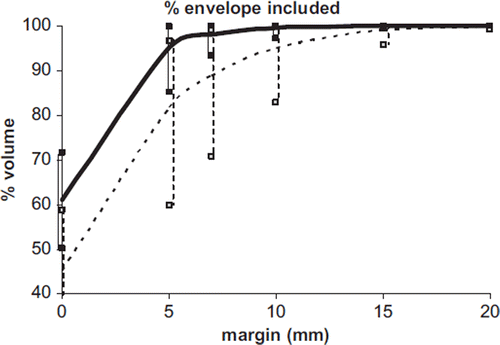
Table II. Margins (in mm) corresponding to 90%, 95%, 98% of coverage probability of the rectal envelope for different reference rectal contours and considering the whole treatment or the second part only (mean values on 10 patients and min-max values, in brackets).
When considering the median rectum of the whole treatment as the reference contour, margins of 10 mm and 15 mm included about 95% of the rectal envelope for seven and 10 patients, respectively. Instead, when considering the second half of treatment, margins of 5 mm and 7mm were adequate for eight and 10 patients, respectively; on average, 95% of the rectal envelope was included by an expansion of 12 mm and 5 mm of the median contours when considering the whole or the second half of the treatment respectively.
When considering the first MVCT or the kVCT rectum as the reference contours, smaller margins were adequate compared to the expansions from the median contour of the whole treatment (). This result is not surprising and depends on the systematically larger rectal volume at the first MVCT/kVCT compared to the average rectum.
For this reason, the corresponding expanded rectal volumes when considering the first fraction as reference are much larger than the corresponding volumes using, for instance, the ninth fraction as reference (on average around 40–60 cm3 for margin of 0.5–1 cm).
Discussion
The current work reports a clear time-trend effect of rectal motion during radiochemotherapy for rectal cancer for a significant proportion of patients.
This result should reasonably extend to GTV as, even if unseen on MVCTs, it is adherent to the rectal wall and should consequently have the same or likely less mobility compared to the rectum.
Interestingly, rectal filling variations measured during the first few fractions of the treatment are consistent with data reported in the prostate cancer scenario; due to this, the practice of emptying the rectum at the planning scan and possibly during therapy is widely recognised as minimising the impact of variable rectal filling during prostate radiotherapy [Citation27]. However, reducing the impact of variable rectal filling during radiochemotherapy of rectal cancer is hardly feasible, due to the local discomfort experienced by the patients.
In any case, our findings suggest that after few fractions it is likely that the irradiation of the whole rectum contributes to a reduction of the ability of rectum to hold air and stool with the consequent “normalisation” of the rectal ampulla.
In the rectal cancer scenario, most reports deal with mesorectum variations and are mostly based on the analysis of few fractions of a conventionally fractionated treatment or in short-course approaches and consequently based on relatively limited statistics [Citation12–14]; these studies have reported systematic large (>10 mm) variations of mesorectum during treatment together with a quite large random variability especially at the anterior border, mainly due to variable bladder filling.
To our knowledge, only two papers have recently dealt with the problem of rectal motion; Briereley et al. [Citation15] investigated local shape variations through elastic registration during a conventional five week, 2 Gy/fr treatment: they analysed data of 17 patients derived from only three CT-scans taken at Weeks 1, 3 and 5 and estimated margins of 8–9 mm to be added to the planning CT-scan by applying a statistical margin recipe. However, the limited statistics of the study resulted in a highly uncertain estimate of these margins.
More recently Chong et al. [Citation16] published a similar investigation on 16 patients treated at 1.8 Gy/fr using cone-beam CT taken during the first 3 fractions and then weekly. They confirmed that the motion of the rectum prevalently concerns the anterior wall of the upper part, but could not report any significant time-trend of rectal volume during the course of the treatment, although a clear volume reduction in several patients was evident; the impact of random variations was probably too high, due to the very few fractions considered.
It is important to underline that the time-trend was not present in all patients of our study, suggesting that a significant proportion of patients are not subject to this phenomenon: looking at the characteristics of the 4/10 patients without trend, no special findings could be observed in terms of location of the tumour, even in its cranial-caudal extension.
Despite the limited number of patients, the availability of daily images permitted the careful monitoring of each daily shape variation and an enhancement of the systematic difference between the rectum at planning kVCT/first fraction and average rectum position during treatment.
It is clear that the availability of a too small sample of the whole treatment (as in the Chong et al and Brierley et al. studies) increases the probability that the random noise of rectal volume variations could hide the trend.
The contouring of just the upper portion of rectum also probably helped demonstrate this effect thanks to the reduction of the impact of the uncertainty in delineating the rectum, which is much greater in the caudal portion (i.e. the anal canal) on CT images [Citation20], and is expected to be higher still when using CBCT or MVCT: a larger contouring uncertainty increases the “noise” in the assessment of volume variations, such that the inclusion of the lower part of the rectum would only add noise without any significant information (as the lower rectum is much more stable than the cranial part).
It is worth mentioning that the reduced mobility in the second half of the treatment and consequently the possibility to apply a smaller margin to GTV, strongly supports our idea of condensing the boosting dose to the residual GTV in the last part of the treatment, also exploiting the tumour shrinkage.
Applying a 6–7 mm margin for the second part of the treatment is sufficient to cover the rectum with at least 90% probability for all patients (); margins of 8–9 mm would be required to cover it with at least 95% probability.
Importantly, the application of soft-tissue registration could further reduce the geometrical uncertainty of the adaptive boost delivery; however, in our large experience in daily IGRT, non-rigid deformations (as for the rectum) are generally not easily counteracted by rigid translations. Thus, in our opinion, the possibility to further reduce the impact of random rectal motion just due to the use of soft-tissue in place of bone registration during adaptive boost seems to be minimal: in any case, the here reported margin estimates remain redundant.
The limitations of our method in estimating margins are clear as we used only the expanded volumes of reference contours and their overlap with the envelopes to assess margins; however, the method is intrinsically redundant, such that the margins found are intrinsically “redundant”. On the other hand, as the rectum is not a rigid body, the application of margin recipes (working well for rigid translations [Citation26]) is quite rough. We need different ways of taking local deformations into account, and our strategy represents one of the simplest and safest ways. Of course, we can better refine our findings with more complex analysis: for instance, our next step (now in progress) is the three-dimensional (3D) analysis of local shape variations, including non-isotropic effects, which are known to be important for rectal motion [Citation12–16].
However, in our opinion, the main results here reported with the relatively simple method of the expanded volumes/envelope ratios are valid and of high value, although the prevalence of male over female patients (nine vs. one) would prospectively suggest the reliability of our margin estimates on a larger population, focusing on just the “adaptive boosting” phase of the treatment.
Importantly, in the case the boosted volume would include the involved mesorectal fascia, our findings are still of interest as the motion of the mesorectum surrounding the GTV is expected to depend mainly on rectal motion [Citation13].
After completing the present study, showing the reduced rectal volume/mobility in the second half of the treatment, we defined a schedule for ART, concentrating the dose escalation in the last 6 fractions: the daily dose to the residual GTV (defined on half-treatment MRI) was increased to 2.9 Gy/fr and subsequently to 3.1 Gy/fr, while keeping the daily dose to CTV (minus GTV) at 2.3 Gy/fr. The results of this pilot ART study will also be the focus of a future investigation.
Another important point is the impact of our findings on the possibility of applying a much larger dose escalation on the residual GTV.
Interest in a completely non-surgical approach to rectal cancer is increasing, although the issue remains controversial.
In any case, in the case of “large” dose escalation without immediate surgery, the risk of late rectal toxicity may be a primary problem, as a proportion of patients would be candidate to salvage surgery. In particular, in case of GTV not involving the whole rectal diameter, the application of a small margin could significantly improve rectum sparing.
This issue should be better investigated in specific planning studies and subsequently tested in controlled clinical trials.
Conclusions
In conclusion, we can state that:
a significant reduction of rectal volume was found in the second part of the treatment for most patients.
Rectal mobility was found to be quite modest in the second half of the treatment.
As a consequence, relatively small margins (6–7 mm) may be used around the residual tumour volume when adaptive boost is delivered in the second half of the treatment and support our idea of concentrating the boosting dose (in a concomitant boost approach) in the last part of the treatment, exploiting GTV shrinkage during the treatment.
Due to the non-isotropic motion of the rectum, margins could also be smaller in correspondence to some portions of the rectum: to better investigate this point, a 3D analysis of local shape variations is in progress.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, . Multidisciplinary rectal cancer management: 2nd European rectal cancer consensus conference (EURECA-CC2). Radiother Oncol 2009;92:148–63.
- Valentini V, Glimelius B. Rectal cancer radiotherapy: Towards European consensus. Acta Oncol 2010;49: 1206–16.
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351: 1731–40.
- Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, . Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99–107.
- Viani GA, Stefano EJ, Soares FV, Afonso SL. Evaluation of biologic effective dose and schedule of fractionation for preoperative radiotherapy for rectal cancer: Meta-analysis and meta-regression. Int J Radiat Oncol Biol Phys 2011;80: 985–91.
- Mohiuddin M, Winter K, Mitchell E, Hanna N, Yuen A, Nichols C, . Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 2006;24:650–5.
- Guerrero-Urbano MT, Henrys AJ, Adams EJ, Norman AR, Bedford JL, Harrington KJ, . Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 2006;65:907–16.
- De Ridder M, Tournel K, Van Nieuwenhove Y, Bijdekerke P, Fierens Y, Duchateau M, . Phase II study of preoperative helical tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:728–34.
- Slim N, Fiorino C, Passoni P, Ronzoni M, Bencardino K, Ricci V, . Preoperative moderately hypofractionated radiotherapy with image-guided tomotherapy concomitant to chemotherapy in rectal cancer adenocarcinoma: A non randomized comparison with hyperfractionated radiotherapy, Radiother Oncol 2010;96(Suppl 1);S288.
- Fuller CD, Nijkamp J, Duppen JC, Rasch CRN, Thomas Jr CR, Wang SJ, . Prospective randomized double blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. Int J Radiat Oncol Biol Phys 2010;79:481–9.
- Tournel K, De Ridder M, Engels B, Engels B, Hoorens A, Everaert H, . Assessment of intrafractional movement and internal motion in radiotherapy of rectal cancer using megavoltage computed tomography. Int J Radiat Oncol Biol Phys 2008;71:934–9.
- Nuyttens JJ, Robertson JM, Yan D, Martinez A. The variability of the clinical target volume for rectal cancer due to internal organ motion during adjuvant therapy. Int J Radiat Oncol Biol Phys 2002;53:497–503.
- Njikamp J, de Jong R, Sonke JJ, Remeijer P, van Vliet C, Marijnen C, . Target volume shape variation during hypo-fractionated preoperative irradiation of rectal cancer patients. Radiother Oncol 2009;92:202–9.
- Ippolito E, Mertens I, Haustermans K, Gambacorta MA, Pasini D, Valentini V. IGRT in rectal cancer. Acta Oncol 2008;47:1317–24.
- Brierley JD, Dawson LA, Sampson E, Bayley A, Scott S, Modeley JL, . Rectal motion in patients receiving preoperative radiotherapy for carcinoma of the rectum. Int J Radiat Oncol Biol Phys 2011;97:97–102.
- Chong I, Hawkins M, Hansen V, Thomas K, McNair H, O'Neill B, . Quantification of organ motion during chemoradiotherapy of rectal cancer using cone-beam computed tomography. Int J Radiat Oncol Biol Phys 2011;81: e431–8.
- Seierstad T, Hole KH, Saelen E, Ree AH, Flatmark K, Malinen E. MR-guided simultaneous integrated boost in preoperative radiotherapy of locally advanced rectal cancer following neoadjuvant radiotherapy. Radiother Oncol 2009;93:279–84.
- Habr-Gama A, Perez RO, Guilherme PSJ, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: A critical evaluation. Sem Radiat Oncol 2011;21: 234–9.
- Fiorino C, Alongi F, Broggi S, Cattaneo GM, Cozzarini C, Di Muzio N, . Physics aspects of prostate tomotherapy: Planning optimization and image-guidance issues. Acta Oncol 2008;47:1309–16.
- Foppiano F, Fiorino C, Frezza G, Greco C, Valdagni R. The impact of contouring uncertainty on rectal 3D dose-volume data: Results of a dummy run in a multicenter trial (AIROPROS01 - 02). Int J Radiat Oncol Biol Phys 2003;57: 573–9.
- Stasi M, Munoz F, Fiorino C, Pasquino M, Baiotto B, Marini P, . Emptying the rectum before treatment delivery limits the variations of rectal dose–volume parameters during 3DCRT of prostate cancer. Radiother Oncol 2006;80:363–70.
- Hoogeman MS, van Herk M, de Bois J, Muller-Timmermans P, Koper PCM, Lebesque JV. Quantification of local rectal wall displacements by virtual rectum unfolding. Radiother Oncol 2004;70:21–30.
- Dice LR. Measures of the amount of ecologic association between species. Ecology 1945;26:297–302.
- Kouwenhoven E, Giezen M, Struikmans H. Measuring similarity of target volume delineations independent of the number of observers. Phys Med Biol 2009;54:2863–73.
- Carillo V, Cozzarini C, Maggiulli E, Fellin G, Rancati T, Valdagni R, . Inter-observer variability in contouring the penile bulb on CT images for prostate cancer treatment planning. Int J Radiat Oncol Biol Phys 2012 (in press).
- Stroom JC, De Boer HC, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys 1999;43:905–19.
- Fiorino C, Di Muzio N, Broggi S, Cozzarini C, Maggiulli E, Alongi F, . Evidence of limited motion of the prostate by carefully emptying the rectum as assessed by daily MVCT image guidance with helical tomotherapy. Int J Radiat Oncol Biol Phys 2008;71:611–7.