Abstract
Background. The aim of this study is to evaluate local control rates after stereotactic body radiotherapy (SBRT) in recurrent spinal metastasis after external beam radiotherapy (EBRT) and new spinal metastatic lesions. Material and methods. Retrospective review of medical records and radiological data was performed on 54 retreatment and 131 initial SBRT patients. To compare various fractionation schedules, the biologically effective dose (BED) was applied. SBRT dose was calculated with linear-quadratic model and normalized to a 2-Gy equivalent dose (nBED, α/β =2 Gy for spinal cord, α/β =10 Gy for tumor). Doses to a point within the spinal cord that received the maximum dose (Pmax) were checked. Local control failure was defined as progression by imaging study. Overall survival, progression free survival, delivered radiation dose to tumor and spinal cord, and spinal cord Pmax nBED were compared in two groups. Results. The mean delivered radiation doses to tumor margin during SBRT were 51.1 Gy2/10 (retreatment) and 50.7 Gy2/10 (initial treatment). Mean survival was 29.6 months (overall)/20.7 months (retreatment)/ 32.4 months (initial treatment). Mean progression free period was 23.9 months (overall)/18.0 months (retreatment)/ 26.0 months (initial treatment). Radiological control rates of retreatment and initial treatment group were 96%/95% at six months, 81%/89% at 12 months and 79%/90% at 24 months. Among 54 retreatment lesions, 13 lesions showed local control failure during follow-up. With regard to spinal cord radiation dose during SBRT, Spinal cord Pmax nBED was 46.2 Gy2/2 (retreatment) and 48.7 Gy2/2 (initial treatment). In retreatment group, total nBED to spinal cord was a mean of 83.4 Gy2/2. There was no case of radiation myelopathy detected. Conclusions. Retreatment of spinal metastases using SBRT provided effective local control without neurological complications.
In the management of spinal metastasis, conventional external beam radiation therapy (EBRT) has been the primary treatment option. A review on palliative EBRT for painful bone metastasis including spine demonstrated an overall response rate of 60% and a complete response rate of 30% [Citation1]. Many patients develop recurrent symptoms such as pain or neurological deficits after EBRT. In patients who are expected to survive long and experience symptoms of relapse after conventional EBRT, re-irradiation of spinal metastasis at greater biological effective dose than the previous radiation may be required to achieve tumor control. However, local recurrence within a previously irradiated field presents a therapeutic challenge because the radiation tolerance of the spinal cord precludes additional delivery of tumoricidal doses of radiation with conventional technique. As such, retreatment radiation can be performed with the technique of modified linear accelerator, Intensity Modulated Radiation Therapy (IMRT), Fractionated Stereotactic Conformal Radiotherapy (FSCT), helical tomotherapy, and SBRT including Cyberknife [Citation2–6].
Owing to advances in radiation delivery technology, it is now possible to deliver ablative radiation doses to spinal metastatic disease safely and effectively in previously irradiated patients. Although data from the literature suggest early onset and longer duration of pain relief, and high rate of local control with SBRT for untreated spinal metastases [Citation7,Citation8], retreatment result of spinal metastasis using SBRT remains scarce. Re-irradiation of spinal tumors using SBRT was performed in some institutes, and their results showed a meaningful interval of local control with improved symptoms and safety [Citation3,Citation4,Citation7,Citation9–11]. Many authors have reported pain relief and neurological symptom improvement after re-irradiation, but not radiological response. The guidelines for spinal cord tolerance in re-irradiation treatment were different between authors and optimal dose for spinal cord re-irradiation remains unestablished.
The aim of this study was to evaluate local control rates in patients who received SBRT due to the recurrences of pre-irradiated vertebral metastases and those in patients who received SBRT for newly developed spinal metastases.
Material and methods
SBRT process using the Cyberknife has been previously described in detail [Citation8]. The planning target volume (PTV) was contoured according to gross tumor volume (GTV) plus a 2–3 mm margin (GTV +2–3 mm = PTV). The spinal cord margin was consisted of the dura mater contoured at the level of the tumor. Similarly, for the cauda equina the dural sac margin represented neural margin. To compare various fractionation schedules, the biologically effective dose (BED) was calculated. SBRT dose was calculated to 2-Gy equivalent normalized BED (nBED). The nBED was calculated by dividing BED by (1 + d/α/β), where d is 2 Gy, and for spinal cord late effect α/β is 2 (Gy2/2), and for tumor tissue early effect, α/β is 10 (Gy2/10). The nBED is analogous to the equivalent EBRT dose in 2-Gy fractions. The total nBED was calculated by adding the nBED of the first course of radiation (EBRT) to the nBED of the second (SBRT). For conventional EBRT, the nBED of the spinal cord were considered to be homogenous.
A retrospective review of medical records and radiological data was performed on 185 patients who underwent SBRT due to metastatic spinal tumors. From June 2002 to December 2008, a total of 185 spinal SBRT was done in 142 patients with spinal metastasis at the Korea Cancer Center Hospital. The study cohort included the cases in which: 1) newly diagnosed symptomatic spinal metastasis; 2) asymptomatic or symptomatic progressed spinal metastasis after prior irradiation; 3) one or two consecutive vertebral bodies were involved; 4) spinal canal encroachment was less than 25%; 5) motor function was greater than Grade 4; and 6) expected survival was longer than three months.
Among them, 54 tumors were retreatment lesions (retreatment group) and 131 tumors were newly developed lesions (initial treatment group). Radiosensitivity of the primary cancer was classified as radiosensitive, moderate and radioresistant. Renal cell cancer and hepatocellular carcinoma were deemed radioresistant, breast cancer and prostate cancer were classified as radiosensitive, and the remaining pathologies as moderate. Lesion level was classified into spinal cord and cauda equina levels. Lesions above T12 vertebral body level belonged to the spinal cord level and lesions below L1 vertebral body level were classified as the cauda equina level.
All treatment plans were reviewed and dose- volume histogram (DVH) data collected. Local control was assessed by follow-up radiological image. Therefore, local control failure means evidences of mass regrowth on magnetic resonance imaging (MRI) or reappearance of hypermetabolic lesion on positron emission tomography CT (PET-CT). Regarding follow-up imaging, MRI or PET-CT was performed at three, six, and 12 months after SBRT. After one year, MRI or PET-CT would be performed every six months. Pain and neurologic status were also investigated by retrospective review. If more than one tumor per patient had been treated at different times, survival and local control would be evaluated for each tumor separately. Overall survival, local-progression free survival, and factors affecting local control were investigated.
Statistical analysis
Progression free period for each tumor was defined from the date of completion of SBRT to last known documented tumor status. Descriptive statistics were shown as mean and median value for continuous variables, and frequencies and proportions for categorical variables. Student's t-test and the Mann-Whitney non-parametric test were used for continuous variables and the χ2 and Fisher's exact test were used for categorial variables. Univariate and multivariate analyses were performed to identify prognostic factors affecting local control by using the Cox proportional hazard model. Overall survival and progression free survival were calculated according to the Kaplan-Meier method. Time-to-progression for the retreatment and initial treatment groups was compared and differences between survival curves were analyzed with the log-rank test. A p-value <0.05 was considered to be statistically significant. All the analyses were performed by using SPSS 16.0 (Chicago, IL, USA).
Results
Patient characteristics and tumor types were presented in . There was no statistical difference in age, radiosensitivity of primary cancer histology, distribution of lesion level, and radiation dose delivered to tumor between retreatment and initial treatment groups. In retreatment group, the mean EBRT dose was 39.2 Gy2 and total radiation dose to tumor was 90.3 Gy2/10 (). However, regarding tumor volume and proportion of patients with epidural mass, significant difference was noted between two groups.
table I. Demographic characteristics and radiotherapeutic parameters (N = 185).
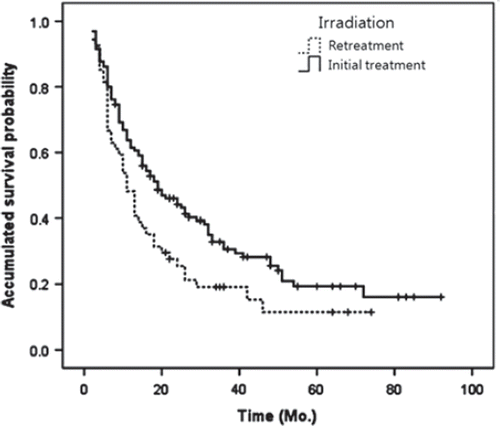
The overall follow-up period for the cohort was 21.8 months. Mean follow-up periods in the retreatment and initial treatment groups were 17.3 months and 23.7 months (p =0.047). The mean overall survival (OS) of the entire study cohort was 29.6 months. In the OS, mean values in the retreatment and initial treatment groups were respectively, 20.7 months and 32.4 months, and median values were respectively, 11.0 months and 19.0 months (p =0.022) ().
Figure 1. Survival curve in the retreatment and the initial treatment groups; mean value was 20.7 months and 32.4 months in each group, which showed statistical difference (p = 0.022).
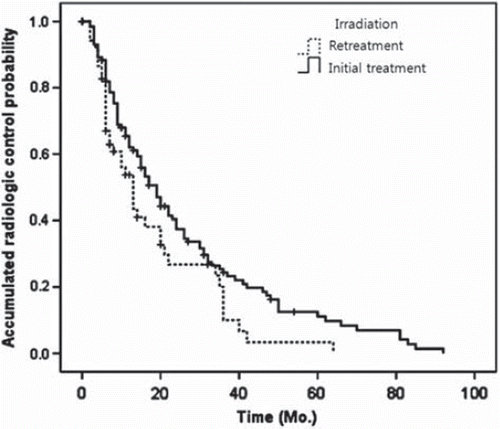
Mean progression free period (PFP) was 23.9 months in the entire study cohort. Mean PFPs in the retreatment and initial treatment groups were respectively 18.0 months and 26.0 months and median PFPs were 13.0 months and 19.0 months (p =0.03) (, ). Pain control rate was 86/93% at six months, 81/89% at 12 months, and 86/90% at two year follow-up. Radiological control rate was 96/95% at six months, 81/89% at 12 months, and 79/90% at two-year follow-up. Local control rates evaluated at specific time points were not different between the two groups (). Regarding prognostic factors for local control, tumor volume and the presence of epidural mass were identified as significant factors on univariate analysis (p =0.024, 0.026). However, on multivariate analysis, only the presence of epidural mass was found to be significant (p =0.002).
Table II. Follow-up result in the retreatment SBRT and the initial treatment groups (N = 185).
Figure 2. Progression free probability curve of the retreatment and the initial treatment groups; mean value was 18.0 months and 26.0 months in each group, which was statistically different (p = 0.03).
Among 54 lesions that were re-irradiated with SBRT, 13 lesions showed local control failure on follow-up. When these 13 patients were compared with remaining 41 patients, no factor was found to correlate to local control failure. Analysis was performed on age, radiosensitivity of the primary pathology, ratio of lesion level (spinal cord vs. cauda equina level), the fraction of patients with epidural mass, interval between EBRT and SBRT, post-SBRT survival, tumor volume, radiation dose in SBRT, and total radiation dose ().
Table III. Comparison between local control failure cases and controlled cases in retreatment group (N = 54).
With regards to radiation dose delivered to the spinal cord during SBRT, maximal point dose (Pmax) nBED was 46.2 Gy2/2 (retreatment group) and 48.7 Gy2/2 (initial treatment group), which showed no statistical significance. When EBRT dose and SBRT spinal cord maximal dose were summated in the retreatment group, the mean total radiation dose to the spinal cord was 83.4 Gy2/2 (). Regarding radiosurgical toxicity, patients were assessed for toxicity using the National Cancer Institute Toxicity Criteria, version 2.0. Toxicity was commonly grade 1 or 2 fatigue in acute period. Late delayed neurological complications were not noted. Symptomatic vertebral compression fractures following SBRT were found in 12 lesions. Symptom was relieved with vertebroplasty in five lesions and with conservative treatment in the remaining lesions.
Discussion
A few publications are available with regard to retreatment spinal SBRT for recurrent spinal metastases after previous irradiation [Citation2–4,Citation11–15]. The first retreatment trial was conducted with modified linear accelerator technique by Hamilton et al. In five patients, local control rate was 100% over a median follow-up period of six months [Citation2]. The largest series of retreated tumors (344 tumors) was published by Gerszten et al., who reported a local tumor control rate of 88% over a median follow-up period of 21 months [Citation7,Citation10]. As reported, current studies on retreatment with spinal SBRT for recurrent spinal metastasis showed 60–100% local control rate over a follow-up period of 6–21 months () [Citation2–4,Citation10,Citation11]. In our series, a mean of 20.6 Gy (single session equivalent dose) was delivered for retreatment group. As a result, PFP was 18.0/13.0 months (mean/median). This result suggests that retreatment spinal SBRT can confer significant benefit of local control on patients with recurrent spinal metastasis. Compared to the initial treatment group, retreatment group experienced poorer PFP. As yet, there is no evidence to suggest that local control rate after spinal SBRT in retreatment group is different from that in initial treatment group. Also in brain metastases, the argument that radiosurgery applied to previously irradiated lesions results in poorer outcome than that in unirradiated cases was not confirmed [Citation9,Citation16].
Table IV. Current studies on spinal SBRT with reported local control rates for patients with image-based tumor progression and previous irradiation.
In most publications on SBRT for the treatment of vertebral metastasis, treatment result was usually determined by clinical symptoms and/or imaging studies. However, four studies that determined tumor local control according to progression by imaging did not consider symptomatic progression as a failure [Citation5,Citation7,Citation10,Citation13]. Chang et al. reported a one-year progression free rate of 84% [Citation13] and Yamada et al. showed 81% of local control rate based on median follow-up of seven months [Citation5]. In our study, the primary end point was image-based tumor progression without consideration of pain status. When SBRT is performed for asymptomatic vertebral metastasis, evaluation of SBRT effect should be made with imaging studies. Particularly in patients whose tumors are confined to the vertebral body without epidural component, MRI cannot be used for the evaluation of treatment result. Instead, PET-CT or bone scan with which metabolic activity can be measured should be used.
Spinal cord radiation tolerance was estimated to be 45–50 Gy over 22–25 fractions [Citation17,Citation18]. In reirradiation of spinal cord, Nieder et al. estimated a risk less than 3%, when a total BED2/2 was less than 135.5 Gy2/2, interval was longer than six months between first irradiation and second one, and a limit of each irradiation was less than 98 Gy2/2 [Citation19]. In Nelson et al.'s publication, they limited the SBRT dose, calculating the time-discounted prior BED to the spinal cord by assuming dose recovery of 25%, 33%, and 50% at six months, one year, and two years, respectively. And they assumed spinal cord tolerance of 59 Gy in 2 Gy/fraction (BED =121.5 Gy2) [Citation14].
Radiation myelopathy (RM) following initial SBRT has been reported by some authors. Sahgal et al. reported five cases, in which Spinal cord Pmax doses were nBED of 33.8, 49.5, 61, 94.5 and 95 Gy2/2 [Citation20]. Ryu et al. reported one case, in which Spinal cord Pmax was 14.6 Gy in a single session (nBED of 60.7 Gy2/2) [Citation21]. In comparison to SBRT, Macbeth et al. reported RM cases following homogenous hypofractionated radiotherapy [Citation22]. Five cases of RM were noted after thoracic radiotherapy in 1048 lung cancer patients. Three patients developed RM following 17 Gy in 2 fractions (nBED of 45 Gy2/2) and two cases with 39 Gy in 13 fractions (nBED of 48.8 Gy2/2). They found no RM cases in 114 patients with 10 Gy in 1 fraction (nBED of 30 Gy2/2). Their data suggest that exceeding 10 Gy in a single fraction to the spinal cord has some risk of RM, which has been widely accepted as a safe guideline for spinal cord protection in SBRT planning.
Several authors reported RM cases after retreatment SBRT and proposed guidelines based on their data. In Gibbs et al.'s series, two previously irradiated patients (25.2 Gy and 40 Gy) developed RM after SBRT, in which SBRT dose was nBED of 46–81 Gy2/3 [Citation8]. Choi et al. reported one case of T5 level RM in 51 lesions of retreatment SBRT series [Citation11]. In that case, total spinal cord Pmax was nBED of 88.9 Gy2/3. Saghal et al. analyzed total Pmax nBED of spinal cord in patients who received retreatment SBRT and found difference between those who developed RM and those who did not [Citation23]. The SBRT re-irradiation spinal cord mean Pmax nBED was 20.0 Gy2/2 in non-myelopathy patients and 67.4 Gy2/2 in myelopathy patients. The mean total spinal cord Pmax nBED was 62.3 Gy2/2 in non-myelopathy patients and 105.8 Gy2/2 in myelopathy patients. Based on their data, they proposed a guideline for retreatment SBRT in terms of spinal cord Pmax nBED. Retreatment SBRT spinal cord Pmax nBED should be less than and 25 Gy2/2 and total spinal cord Pmax nBED should be less than 70 Gy2/2. In our study, retreatment SBRT spinal cord mean Pmax nBED was 46.2 Gy2/2 and mean total spinal cord Pmax nBED was 83.4 Gy2/2. During follow-up, no patients showed RM. Based on our data, in terms of spinal cord Pmax nBED, spinal cord tolerance to radiation appears to be higher than we expected. In addition, BED calculation to combine doses from homogenous radiation used in conventional EBRT and partial volume irradiation of SBRT may not provide correct measurement of spinal cord irradiation.
The size of the tumor is a major determinant of the steepness of the dose gradient and only tumors measuring about 50 cm3 or less can be treated radiosurgically [Citation24,Citation25]. Lesion volumes of our retreatment group ranged from 1.3 cm3 to 265 cm3 with a mean of 58.4 cm3. The reason of high tumor volume was the proportion of the sacral metastasis. The mean tumor volume of sacral metastasis (N =14) was 124 cm3. The mean volume in patients whose tumors were located in the cervical and thoracic levels was 26.5 cm3. In Choi et al.'s 51 cases of retreatment, 90% of all cases were cervical and thoracic metastasis and the median value of tumor volume was 10.3 cm3 (0.2–128.6 cm3) [Citation11]. In another series by Sahgal et al., the mean tumor volume was 21 cm3 (0.4–177 cm3) [Citation10]. In terms of tumor volume, lesions with large volume tend to show local failure after treatment. In Gerszten et al.'s report, local failure was seen in 6/60 cases treated, of which the mean tumor volume was 102.6 ml [Citation26]. In our study population, tumor volumes of the retreatment and the initial treatment groups were 58.4 cm3 and 36.4 cm3. Lesions with large volume frequently accompany epidural mass in the spinal canal. The reason why progression free period in the retreatment group was shorter than that of the initial treatment group was the high proportion of patients with epidural mass. Whether local control can be maintained or not is determined by the minimum distance from the PTV to the spinal cord margin [Citation9,Citation12].
In conclusion, retreatment of spinal metastasis using SBRT appears to provide effective local control without neurological complications. Owing to the high precision of SBRT, radiation distribution is sharply delineated and steeply graded between tumor and the spinal cord. Spinal cord tolerance to retreatment SBRT was variable and was estimated to be slightly higher than previous reports.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423–36.
- Hamilton AJ, Lulu BA, Fosmire H, Stea B, Cassady JR. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery 1995;36:311–9.
- Milker-Zabel S, Zabel A, Thilmann C, Schlegel W, Wannenmacher M, Debus J. Clinical results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003;55:162–7.
- Mahan SL, Ramsey CR, Scaperoth DD, Chase DJ, Byrne TE. Evaluation of image-guided helical tomotherapy for the retreatment of spinal metastasis. Int J Radiat Oncol Biol Phys 2005;63:1576–83.
- Yamada Y, Lovelock DM, Yenice KM, Bilsky MH, Hunt MA, Zatcky J, . Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: A preliminary report. Int J Radiat Oncol Biol Phys 2005;62:53–61.
- Grau C, Hoyer M, Lindegaard J, Overgaard J. The emerging evidence for stereotactic body radiotherapy. Acta Oncol 2006;45:771–4.
- Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine 2007;32:193–9.
- Gibbs IC, Kamnerdsupaphon P, Ryu MR, Dodd R, Kiernan M, Chang SD, . Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol 2007;82:185–90.
- Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: A critical review. Int J Radiat Oncol Biol Phys 2008;71:652–65.
- Sahgal A, Ames C, Chou D, Ma L, Huang K, Xu W, . Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys 2009;74:723–31.
- Choi CY, Adler JR, Gibbs IC, Chang SD, Jackson PS, Minn AY, . Stereotactic radiosurgery for treatment of spinal metastases recurring in close proximity to previously irradiated spinal cord. Int J Radiat Oncol Biol Phys 2010;78:499–506.
- Garg AK, Wang XS, Shiu AS, Allen P, Yang J, McAleer MF, . Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center Experience. Cancer 2011;117:3509–16.
- Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, . Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151–60.
- Nelson JW, Yoo DS, Sampson JH, Isaacs RE, Larrier NA, Marks LB, . Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys 2009;73:1369–75.
- Amdur RJ, Bennett J, Olivier K, Wallace A, Morris CG, Liu C, . A prospective, phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am J Clin Oncol Epub 2009 Jun 12.
- Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, . Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 2000;47:291–8.
- Withers HR, Taylor JM, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys 1988;14:751–9.
- Nakagawa K, Ohkuma K, Yamashita H, Masuda M, Matsumoto Y, Gotoh T. Radiation therapy did not alleviate complete paralysis due to metastasis of lung adenocarcinoma to thoracic vertebrae until four months later. Acta Oncol 2011;50:606–8.
- Nieder C, Grosu AL, Andratschke NH, Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 2006;66:1446–9.
- Sahgal A, Ma L, Gibbs I, Gerszten PC, Ryu S, Soltys S, . Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;77:548–53.
- Ryu S, Jin JY, Jin R, Rock J, Ajlouni M, Movsas B, . Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer 2007;109:628–36.
- Macbeth FR, Wheldon TE, Girling DJ, Stephens RJ, Machin D, Bleehen NM, . Radiation myelopathy: Estimates of risk in 1048 patients in three randomized trials of palliative radiotherapy for non-small cell lung cancer. The Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:176–81.
- Sahgal A, Ma L, Weinberg V, Gibbs IC, Chao S, Chang UK, . Reirradiation HUMAN spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82:107–16.
- Wowra B, Muacevic A, Zausinger S, Tonn JC. Radiosurgery for spinal malignant tumors. Dtsch Arztebl Int 2009;106:106–12.
- Sahgal A, Chuang C, Larson D, Huang K, Petti P, Weinstein P, . Split-volume treatment planning of multiple consecutive vertebral body metastases for cyberknife image-guided robotic radiosurgery. Med Dosim 2008;33:175–9.
- Gerszten PC, Burton SA, Ozhasoglu C, Vogel WJ, Welch WC, Baar J, . Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine 2005;3:288–95.
