Abstract
The combination of irinotecan and bevacizumab has shown efficacy in the treatment of recurrent glioblastoma multiforme (GBM). A prospective, phase II study of 85 patients with various recurrent brain tumors was carried out. Primary endpoints were progression free survival (PFS) and response rate. Material and methods. Patients with recurrent primary brain tumors with performance status 0–2 were eligible. Intravenous bevacizumab 10 mg/kg and irinotecan 125/340 mg/m2 were administered every 14 days. Evaluation was carried out every eight weeks using MRI and Macdonald response criteria. Treatment was continued until progression. Results. In total 85 patients were included with the following histologies: GBM (n = 32), glioma WHO gr. III (n = 33), glioma WHO gr. II (n = 12) and others (n = 8). Patients received a median of four cycles. ORR (overall response rate) for glioblastoma was 25% and 59% achieved stable disease (SD). Median PFS was 5.2 months. For grade III gliomas ORR was 21% and 45% had SD. Median PFS was 3.7 months. No objective responses occurred in grade II gliomas. In the non-glioma population, one PR as well as several long PFS times were observed. Discussion and conclusion. The combination of bevacizumab and irinotecan is well tolerated and moderately efficacious in glioblastoma and glioma WHO gr. III. A majority of patients achieve at least disease stabilization. Prolonged progression-free survival in non-glioma patients warrants further research.
Primary brain tumors are heterogeneous diseases that generally carry poor prognoses.
Gliomas comprise approximately half of these, followed by meningiomas (27%), schwannomas (6–8%), pituitary adenomas (6–8%), medulloblastomas (1–2%) and others (8–10%) [Citation1]. Despite aggressive primary treatment, the rate of relapse for primary brain tumors is high.
Sustained angiogenesis is recognized as a hallmark of cancer [Citation2]. The strategy of inhibiting angiogenesis by blocking the pathway of the vascular endothelial growth factor (anti-VEGF treatment) has proved successful in several solid tumors (e.g. metastatic colorectal cancer [Citation3], lung cancer [Citation4], ovarian cancer [Citation5]), when combined with systemic cytotoxic treatment. It has been hypothesized that the efficacy of anti-angiogenic treatment is due to tumor vessel normalization which in turn improves delivery of the cytotoxic drug [Citation6]. Irinotecan is a camptothecin derivate that inhibits the nuclear enzyme, topoisomerase I. It permeates the blood-brain barrier and has shown single agent anti-tumor efficacy against recurrent glioma in several trials [Citation7]. In combination with the anti-VEGF antibody, bevacizumab, irinotecan has shown promising activity against recurrent glioblastoma multiforme (GBM). Response rates and progression-free survival at six months (PFS/PFS-6) seem improved by this regimen compared with historical results [Citation8–11]. As GBM is a highly vascular tumor [Citation12] this may not be surprising, but attempts to correlate response to bevacizumab treatment with biomarkers of angiogenesis have so far been equivocal (please refer to Discussion). Evaluating the efficacy of bevacizumab in tumors that are less highly vascularized (e.g. recurrent astrocytoma grade II) but lacked other treatment options therefore seemed warranted.
We have previously reported a retrospective analysis of a series of high-grade gliomas and various other primary brain tumors [Citation9] but no prospective studies have addressed the effect of antiangiogenic treatment in low-grade gliomas and non-glial, primary brain tumors.
Thus, a prospective study designed to evaluate the effect of bevacizumab (B) and irinotecan (I) on various recurrent brain tumors, including gliomas, was carried out. The purpose was to prospectively evaluate the efficacy of B + I in primary brain tumors, and compare the outcome measures in GBM and non-GBM, in order to identify and select disease entities that might benefit from this treatment regimen in future prospective trials with more narrow inclusion criteria.
Patients and methods
Patients
Patients with recurrent or progressive primary brain tumors with no other standard therapy available were considered for eligibility. Histological diagnosis was defined by the most recent surgical tissue sample available. All patients had received standard treatment at the time of initial diagnosis. Resection prior to entry in the study was performed as indicated. The study was performed according to the Helsinki Declaration and ICH-GCP and was approved by the local ethics committee (j.nr.H-KF-2006_5905) and registered with ClinicalTrials.gov (NCT 00463203).
Inclusion criteria were as follows: measurable [by computed tomography (CT)/magnetic resonance imaging (MRI) scan] recurrent or progressive disease after standard treatment, ECOG PS 0–2, age > 18, life expectancy > 3 months, normal blood tests (hemoglobin > 6.2 mmol/l, platelets > 125 × 109, leukocytes > 3 × 109, neutrofiles > 1.5 × 109, AST or ALT ≤ 3 × upper normal limit, bilirubin ≤ 1.5 × upper normal limit, creatinine clearance > 45 ml/min, APTT ≤ normal limit, INR ≤ normal limit), use of contraceptives/preservatives when relevant and signed, informed consent.
Exclusion criteria were radiotherapy or chemotherapy within the last four weeks, co-medication that could interfere with study results (e.g. immunosuppressives other than corticosteroids), any condition (medical, social, psychological) that could prevent adequate information and follow-up, any other active malignancy or previous malignancies within the last five years except adequately treated basal or squamous cell carcinoma of the skin or carcinoma in situ, any significant cardiac disease (NYHA class II or greater), arrhythmia, congestive heart failure, acute myocardial infarction within six months or unstable angina pectoris, clinically significant peripheral vascular disease, evidence of bleeding diathesis, coagulopathy or use of ASA, NSAID's or clopidogrel, major surgical procedures, open biopsy or significant traumatic injury within 28 days prior to Day 0 or anticipation of need for major surgical procedure during the course of the study, fine needle aspirations or core biopsies within seven days prior to Day 0, history of abdominal fistula, gastrointestinal perforation or intra-abdominal abscess within six months prior to Day 0, history of known HIV, hepatitis B or C, any ongoing infection, uncontrolled diabetes mellitus, serious non-healing wound, ulcer or bone fracture, pregnancy or breast feeding, requirement of therapeutic anti-coagulation, blood pressure > 150/100 mmHg, grade ≥ 2 proteinuria.
Treatment
A single arm, prospective phase II study was carried out in four participating centers in Denmark. All patients were treated in an out-patient setting every 14 days with intravenous bevacizumab (Avastin, Roche) 10 mg/kg and irinotecan (Campto, Pfizer) 340 mg/m2 or 125 mg/m2 according to use of EIAED (enzyme inducing anti-epileptic drugs) or not, respectively. Two treatments (28 days) comprised one cycle of chemotherapy.
Blood tests, blood pressure and urine dip stick were obtained prior to each treatment. Irinotecan dose was reduced to 80% in case of grade 3–4 toxicity. Dose reduction of bevacizumab was not allowed.
Evaluation was carried out every two cycles (eight weeks) of chemotherapy using the Macdonald criteria [Citation13] which include cross-sectional tumor area on imaging, clinical characteristics and steroid usage. The possible outcomes are CR (complete response), PR (partial response), SD (stable disease) or PD (progressive disease). Only evaluations where a cross sectional area was specified by the radiologist were counted as being valid, although clinical progression without image confirmation could be counted as PD. CR demanded the disappearance of previously visible lesions.
For MRI, T1 and T2 sequences were generated, with and without Gd-contrast. The RANO criteria [Citation14] had not yet been published when this study was conceived.
ECOG performance status was recorded and adverse events were evaluated with the use of the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. On-study treatment consisted of six cycles of therapy. In case of clinical benefit, treatment could continue beyond this until progression, unmanageable toxicity or death occurred.
Sample size justification and statistics
The primary endpoint was response rate and a rate of 40% was considered to be of interest. A response rate < 20% was considered inadequate for continuation. The trial had a power of 90% to detect a response rate of 40% and a 5% chance of continuing with a response rate below 20%. A two-stage design was employed, with the possibility of discontinuation following stage one if predetermined criteria were not met. Thus, an initial cohort of 19 evaluable patients would be treated. Four patients were required to obtain at least a partial response for continuation. Upon meeting this criterion the trial could proceed to the second stage with inclusion of 35 additional evaluable non-GBM patients. Therefore 54 non-GBM patients would be included in all. Concurrently, patients with GBM were to be included in parallel for comparison. Other endpoints included median time to progression, safety parameters and survival.
Response rates were calculated using the intention-to-treat (ITT) population. Confidence intervals were estimated using the exact confidence interval (Clopper-Pearson method). Survival analyses were performed using the Kaplan-Meier log-rank method. Multiple logistic regression analysis was employed to compare co-variates’ influence on response. Comparison of survival times in responders and non-responders was performed using the landmark method. Sixteen weeks after the initiation of therapy was selected as landmark as all responses were recorded at this time point. SPSS version 18.0 was used for all statistical analysis.
Results
A total of 85 patients with a variety of recurrent primary brain tumors were recruited between February 26, 2007 and January 15, 2010. In all, 53 non-GBM- and 32 GBM patients were included in the study. The number of non-GBM patients fell one short of the planned number due to an error in recruitment of the final patient.
Patients’ baseline characteristics are listed in . Gliomas constituted a large majority (77/85 = 91%) of the study population. All glioma patients had previously been exposed to alkylating chemotherapy. Most patients had had one line of previous chemotherapy (84%) whereas 14% had received more than one line of treatment. Two patients with grade III ependymoma and the patients with grade III meningioma- and malignant prolactinoma were chemotherapy naive.
Table I. Patients’ baseline characteristics.
Follow-up time was a minimum of six months for surviving patients (median follow-up = 8.3 months, range for total population: 0.5–44.1 months). Patients received a median of four cycles of chemotherapy (range: 0.5–25). Seventy-nine patients were evaluable at first evaluation. Of the six non-evaluable patients, two died from disease progression before the first evaluation [one GBM and one AA (anaplastic astrocytoma)]. The remaining four patients discontinued due to hematological toxicity (n = 1), CNS hemorrhage (n = 1), stroke (n = 1) or treatment related death.
Responses in glioma population
As seen in , overall response rates (ORR = CR + PR) for GBM, grade III- and grade II gliomas were 25%, 21% and 0%, respectively. For GBM and grade III gliomas, the treatment generally caused radiographic tumor regression. The waterfall plot in demonstrates tumor shrinkage observed in GBM.
Figure 1. Summary of best radiographic responses for the 28 evaluable glioblastoma multiforme. Horizontal grey lines represent 1 25% and 2 50% change from baseline.
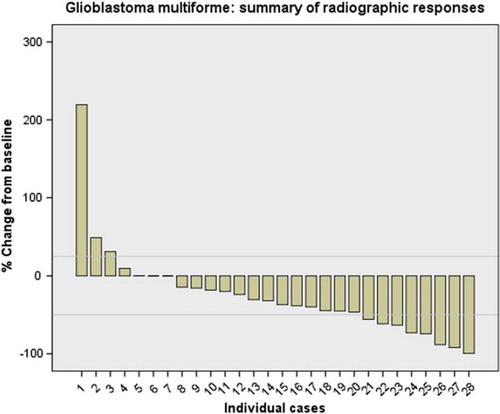
Table II. Clinical responses and outcomes for the glioma population. 95% confidence intervals are noted in parentheses.
Among a total of 16 patients who showed objective responses, 11 of these (69%) occurred at first evaluation after two cycles. One patient had a PR at first evaluation that regressed further to a CR at the next evaluation. The remaining four patients all had ‘minor responses’ (34–45% regression) at first evaluation.
For the seven patients with grade III glioma exhibiting CR or PR, a post-hoc radiological review was performed in order to evaluate the size of non-enhancing lesions on T2 weighted MRI images during the course of treatment. Images at baseline and at time of best response were available in six cases. The cross-sectional area of these lesions generally decreased (range: 4% increase to 62% decrease, median 30% decrease). In one case, T2 weighted images were not acquired at baseline but at subsequent scans. This patient demonstrated progressing lesions on T2 FLAIR images while the contrast enhancing lesion regressed. The overall survival of this patient was 8.3 months which was the shortest OS among the seven responding grade III glioma patients.
Response in non-glioma population
Partial response was achieved in a patient with medulloblastoma, and stable disease was recorded in three patients with prolactinoma, meningeoma and schwannoma, respectively. Long term disease stabilization was observed in the non-glioma patients as shown in .
Table III. Responses and outcomes for individuals in the non-glioma population.
Survival and progression free survival in glioma population
Median overall survival (OS), progression free survival (PFS) and progression free survival at six months (PFS-6) are shown in . A landmark analysis showed that responders with GBM, but not grade III glioma, survived significantly longer than non-responders (median survival 13.4 vs. 4.1 months from landmark for GBM, p = 0.03).
Clinical efficacy of treatment
Of the total of 16 patients with response, 11 (69%) maintained the same performance status (PS) throughout the study. Three patients improved by one grade, whereas two responders worsened by one grade. Of patients with SD (n = 49) and baseline PS 1–2 (n = 31), four (13%) showed improved PS during treatment.
Steroid use was decreased following two cycles of treatment for 67% of responders, 44% of patients with SD and 13% of patients with PD, counting only those with baseline steroid use.
Factors affecting response in high grade glioma (WHO grades III and IV)
A number of clinical parameters were evaluated for possible correlation to treatment response using a multiple logistic regression analysis.
No statistically significant correlation could be found for histopathological grade, tumor size, steroid use at baseline, age, PS, or development of hypertension during treatment. shows the odds ratio estimates and 95% confidence limits for pooled data from grade III and IV glioma. The area under the receiver operating characteristic curve was 0.58 which implies that a model based on these parameters would not be valuable in predicting the likelihood of response.
Table IV. Odds ratio of response for different clinical parameters. No statistically significant associations were found.
Toxicity
Toxicity is listed in . Generally, toxicity was mild but frequent. Fatigue, nausea and diarrhea were the most common grade 1–2 side effects to treatment, each suffered by > 40% of all patients at some point during treatment. Manageable hypertension (grade 1–2) or proteinuria (grade 3–4) was experienced by 18% and 16%, respectively.
Table V. Toxicity. All values indicate percentage of valid data. Only changes from baseline are reported.
There were three deaths that were considered to be treatment related (3.5%). One patient died due to an acute onset cardio-pulmonary failure during bevacizumab administration. Clinically, pulmonary embolism was suspected. Two patients suffered fatal hemorrhages in the CNS and the upper gastro-intestinal tract, respectively. Another patient died of pneumonia and Haemophilus influenzae septicemia with normal WBC. One patient died after having complained of sudden onset of sharp back pain, followed by nausea and dizziness. One patient developed a non-fatal wound dehiscence in the scalp. Another 6% of patients suffered non-fatal (grade 3 - 4) venous thromboembolism. In addition, one patient developed osteonecrosis of the jaw after six months of therapy whereupon bevacizumab was discontinued.
Discussion
Published response rates for the combination of B + I range from 30–68% [Citation8–10]. The response rate of 25% for GBM that we report here may appear lower, but is naturally subject to statistical variation (95% CI 11–43%), as well as interpretational variation.
The PFS and PFS-6 (5.2 months and 28%) seem to compare reasonably well with published data (PFS range: 5.1 months–7.6 months, PFS-6 range: 37–64%) [Citation8–10,Citation15,Citation16].
As for grade III and II glioma, the present data indicate a lower efficacy compared to GBM. No objective responses occurred among the 12 grade II patients, and disease stabilization was of short duration, as evidenced by a low PFS of 5.2 months.
A similar pattern of inferior response for the lower grade tumors was observed in our previously reported retrospective study, where ORR for grade III tumors only reached 15% [Citation9]. Desjardins et al. seem to have obtained superior results in a similarly sized group of patients with grade III tumors demonstrating an overall response rate of 61%, and a PFS-6 of 55% [Citation17]. However, the large difference between the initial response rate published in the BRAIN study (of GBM) [Citation10] and that which was found by the subsequent independent radiological reviews (46% vs. 26%) [Citation18] suggests that interpretational variation may be significant in GBM when using the Macdonald criteria for anti-VEGF treatment. This is likely also to be true of grade III tumors. Updated response criteria have since been suggested (RANO) [Citation14], which might improve inter-observer variability, however, this is yet to be proven. In this study, one of seven responding grade III glioma patients experienced increasing T2/FLAIR lesions. According to the RANO criteria, this is implies progressive disease [Citation14]. Thus, the response rate reported here would be lower (18% vs. 21%) using these criteria and it seems likely that this would be a general tendency in reporting results of trials using anti-VEGF treatment.
While it is generally accepted that bevacizumab treatment for recurrent glioblastoma results in frequent radiological improvement, most notably in the T1 contrast enhanced sequences, it is unknown whether this effect prolongs overall survival. No controlled studies have been performed, and bevacizumab has not been approved by the European Medicines Agency (EMA) for this indication, as it was in the US by the FDA. The ongoing EORTC study 26091 is comparing temozolomide with and without bevacizumab in recurrent grade II and III glioma, and these data are awaited.
In the present study, responders among the GBM clearly achieved longer OS compared to non-responders. These findings are in accordance with the landmark analysis performed by Prados et al. using the BRAIN study data [Citation19]. For grade III glioma, no such correlation was found. Interestingly, this was also the case in the paper published by Desjardins et al. mentioned above, although a non-significant tendency was observed in both sets of data. Ultimately, only controlled trials have the potential to definitively address the question of overall survival benefit using this regimen.
Toxicity with the B + I regimen is not negligible. Apart from the life-threatening side effects consistently reported, a considerable proportion of patients experience grade 1–2 fatigue (49%), nausea (52%) and diarrhea (40%). This is significant in the setting of recurrent disease. It is apparent that predictive biomarkers are needed. Thus far, attempts to identify molecular biomarkers have not been entirely successful. A positive correlation between VEGF immunoreactivity and radiological response was found by Sathornsumetee et al. [Citation20], but Hasselbalch et al. were not able to reproduce these results in a similar sized population where a number of other markers of angiogenesis and hypoxia also were tested (e.g. HIF-1a, HIF-2a and CA-9) [Citation21]. Biomarker analyses of archieval tissue from patients in the present study is planned.
Only eight patients with non-glioma histologies were included in this study. This was slightly disappointing, as one of the main purposes was to evaluate the efficacy of B + I in non-glioma brain tumors. The four participating centers accrue from the whole population of Denmark (approximately 5.5 million inhabitants), which illustrates clearly the difficulty in gaining knowledge of very rare diseases, and the importance of international collaborations.
The patient with medulloblastoma had a PR of moderate duration (6.1 months). This patient subsequently received several lines of experimental treatment, including radionucleide therapy with DOTATATE, which also caused a marked but short-lived response. Only case studies using B + I treatment in medulloblastomas exist; our group found no effect in a previous retrospective study [Citation9]. Cooperative clinical trials are necessary to address this issue further.
The patient with malignant prolactinoma clearly had clinical benefit of the treatment, with PFS close to three years. One similar patient was also reported with a PR in our retrospective analysis [Citation9].
Both gliosarcoma patients treated exhibited ‘minor responses’ with PFS of four to six months. We have been unable to find reports documenting clinical outcomes for gliosarcoma patients at recurrence, but feel that the results obtained warrant further research.
The patient with grade III meningioma had stable disease and received six cycles of treatment. At the end of observation, 19.3 months after enrollment, the disease was still stable. This may be more indicative of indolent disease (initially diagnosed in 1990) than an effect of the treatment.
The effect of bevacizumab for benign schwannoma was recently demonstrated in a series of 10 consecutive patients with neurofibromatosis type II [Citation22], where tumor regression was seen in 9/10 patients, and hearing improvement seen in 4/7 eligible patients. VEGF was found to be expressed in all tumors. Likewise, the one schwannoma patient in our study showed a minor response and a moderate PFS of 6.8 months. Further research seems warranted in this group.
One ependymoma patient had a PD and another had SD (47% reduction of tumor) lasting 14.5 months. A retrospective analysis from 2009 of eight ependymoma patients treated with B showed an encouraging ORR of 75% [Citation23]. A prospective study seems to be warranted.
Conclusion
The combination of bevacizumab and irinotecan causes tumor regression in a majority of patients with recurrent grade IV and III glioma. Macdonald responses were seen in 25% and 21% of patients, and PFS was 5.2 months and 3.7 months, respectively. A landmark analysis at the second evaluation indicates a clinically meaningful survival advantage (OS and PFS) for responders over non-responders for GBM, but not for grade III tumors. The regimen seemed less active for grade II glioma, where no objective responses occurred.
In non-glioma patients, one PR occurred (medulloblastoma) and promising ‘minor responses’ with long PFS times were seen in several cases. Prospective studies for these diseases seem warranted but may be difficult to carry out due to their rare occurrences. International collaboration will be necessary.
Acknowledgements
We thank Ib Jarle Christensen at the Finsen Laboratory for assistance with the statistical analyses and Peter Gideon at the Department of Radiology, Rigshospitalet, for assistance with the radiological review. Preliminary results of this study were presented in poster form at the 2010 Annual Meeting of the Society for Neuro-Oncology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Danish Neuro-Oncology Group. 2009 Annual Report of the Danish Neuro-Oncology Registry.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, . Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, . A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96.
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005;307:58–62.
- Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol 2009;11:80–91.
- Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, . Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253–9.
- Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, . Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 2009;48:52–8.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27: 4733–40.
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, . Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999;17:2572–8.
- Jain RK, di TE, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci 2007;8:610–22.
- Macdonald DR,, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80.
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, . Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72.
- Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Ellika S, . Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol 2009;91: 329–36.
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, . Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–9.
- Desjardins A, Reardon DA, Herndon JE, Marcello J, Quinn JA, Rich JN, . Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 2008;14: 7068–73.
- Wick W, Weller M, van den BM, Stupp R. Bevacizumab and recurrent malignant gliomas: A European perspective. J Clin Oncol 2010;28:e188–9.
- Prados M, Cloughesy T, Samant M, Fang L, Wen PY, Mikkelsen T, . Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 2011;13:143–51.
- Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, McLendon RE, Desjardins A, . Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 2008;26:271–8.
- Hasselbalch B, Eriksen JG, Broholm H, Christensen IJ, Grunnet K, Horsman MR, . Prospective evaluation of angiogenic, hypoxic and EGFR-related biomarkers in recurrent glioblastoma multiforme treated with cetuximab, bevacizumab and irinotecan. APMIS 2010;118:585–94.
- Plotkin SR, Stemmer-Rachamimov AO, Barker FG, Halpin C, Padera TP, Tyrrell A, . Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 2009;361:358–67.
- Green RM, Cloughesy TF, Stupp R, DeAngelis LM, Woyshner EA, Ney DE, . Bevacizumab for recurrent ependymoma. Neurology 2009;73:1677–80.
