Abstract
Introduction. The aim of the present study was to investigate the impact of age at diagnosis on prognosis in patients treated with curatively intended radiotherapy for NSCLC. Material and methods. This is a joint effort among all the Swedish Oncology Departments that includes all identified patients with a diagnosed non-small cell lung cancer that have been subjected to curatively intended irradiation (≥50 Gy) treated during 1990 to 2000. Included patients had a histopathological/cytological diagnosis date as well as a death date or a last follow-up date. The following variables were studied in relation to overall and disease-specific survival: age, gender, histopathology, time period, smoking status, stage and treatment. Results. The median overall survival of all 1146 included patients was 14.7 months, while the five-year overall survival rate was 9.5%. Younger patients (<55 years), presented with a more advanced clinical stage but had yet a significantly better overall survival compared with patients in the age groups 55–64 years (p = 0.035) and 65–74 years (p = 0.0097) in a multivariate Cox regression analysis. The overall survival of patients aged ≥75 years was comparable to those aged <55 years. Conclusion. In this large retrospective study we describe that patients younger than 55 years treated with curatively intended radiotherapy for NSCLC have a better overall survival than patients aged 55–64 and 65–74 years and that younger patients seem to benefit more from the addition of surgery and/or chemotherapy to radiotherapy. Due to the exploratory nature of the study, these results should be confirmed in future prospective trials.
The incidence of lung cancer has increased throughout the 20th century and is today the most common cancer in the western world [Citation1]. Lung cancer is divided into two major histological subtypes, non-small cell lung cancer (NSCLC) accounting for more than 80% of all lung cancer cases and small cell lung cancer (SCLC) accounting for less than 20% of all lung cancer cases [Citation2]. The median age at diagnosis is 68 years for patients with non-small cell lung cancer (NSCLC) and 20% of lung cancer deaths occur in patients aged 80 or more [Citation3]. Since the number of elderly patients has increased dramatically in the industrialised world [Citation4] there is a belief that the scientific society should look into the differences between these patients’ physiological reserve, organ function, and drug pharmacokinetics in comparison with younger patients and address this in clinical trials [Citation5]. Unfortunately, the number of randomised studies taking this into account is limited since many trials exclude patients who are aged over 70 years. In the present study, we have retrospectively investigated 1146 patients with NSCLC, treated with curatively intended radiotherapy (≥50 Gy) in Sweden during 1990–2000 with the aim to elucidate whether the age at diagnosis can predict the response to treatment, a question that according to our knowledge has not previously been addressed in this context.
Material and methods
The present study is a joint effort among all the Swedish Oncology Departments. Included in the study are patients with a diagnosed non-small cell lung cancer that have been subjected to curatively intended irradiation (≥50 Gy) and treated during the years 1990 to 2000. The present study was reviewed and approved by the research ethical committee (Dnr 2005: 025). The included patients were localised by a manual search of all oncology departments’ medical records and radiation charts. A reference group composed of five oncologists visited all the collaborating hospitals and reviewed the charts together with the medically responsible physician for the treatment of lung cancer at the specific site. In the analyses, all patients were included who had either a histopathological or a cytological diagnosis date as well as a death date or a last follow-up date. The following variables were collected: age, gender, time period, smoking status, symptoms at presentation (hoarseness, haemoptysis, thoracic pain and/or pneumonia), performance status at the start of treatment, histology (defined as squamous cell carcinoma, adenocarcinoma or other non-small cell histology), stage (which was re-evaluated by three of the authors based on available information in the charts as well as based on available x-ray investigations), treatment (first line as well as second line treatment) and occurrence of relapse.
Data were missing for some patients regarding some of these variables. However, these patients were not excluded from the study unless lack of data required to estimate survival. This, unfortunately, causes inconsistencies among some of the frequencies accounted for in the results section of this article.
Statistics
Patient characteristics at diagnosis are presented using standard descriptive statistics. Overall survival and disease-specific survival were analysed with Kaplan-Meier product-limit estimates. Survival curves for different categories were compared using the log-rank test. The follow-up time was calculated from the date of diagnosis to death or last follow-up until the end of 2008. Age was defined as age at diagnosis. For disease-specific survival, death from other known causes than lung cancer was considered a competing risk [Citation6] Thus, patients who died from other known causes were analysed as censored for this variable and patients for whom cause of death was unknown were excluded from the analysis of disease-specific survival. Overall survival was also analysed using Cox proportional hazards regression models. Univariate and multivariate analyses were performed. The multivariate model was adjusted by gender and age at diagnosis. Results are presented as hazard ratios with 95% confidence intervals (95% CI). In addition p-values were given, where p < 0.05 was regarded as statistically significant.
Results
Patient characteristics
A total of 1146 patients with non-small cell carcinoma were included and of these, 391 (34%) were women. The median age and range was 65 (25–87) years. The age distribution was as follows: < 55 years: 222 patients (19%), 55–64 years: 335 patients (29%), 65–74 years: 422 (37%) patients and ≥ 75 years: 167 patients (15%). The percentage of women was highest in the youngest age group (52%) and decreased with increasing age to 25% in patients aged ≥ 75 years. At diagnosis, the percentage of non-smokers showed very little variation among the age groups whereas the percentage of current smokers was highest in the youngest age group (70%) and decreased with increasing age to 40% in the oldest age group. Among the four defined symptoms at presentation (hoarseness, haemoptysis, thoracic pain and pneumonia), thoracic pain seemed to decrease with increasing age, whereas the opposite was observed for haemoptysis. Hoarseness and pneumonia showed little variation with age. The performance status according to WHO (grade 0 3 in the present study) was fairly similar in the different age groups with an absolute majority of patients being classified as WHO 0 1. Higher performance status grades were more common with increasing age; 6.2% of patients aged < 55 were classified as WHO 2 3 as compared with 13% of patients aged ≥ 75 years. Concerning histopathology, the proportion of adenocarcinomas decreased with increasing age from 37% in the patients aged < 55 to 17% in patients aged ≥ 75 years. The opposite trend was observed for squamous cell carcinomas which showed an increase from 40% in patients aged < 55 to 68% in patients aged ≥ 75 years. For the 785 patients, where information concerning cause of death was available, 731 (93%) died from lung cancer while 54 (7%) died of other reasons. The cause of death according to the clinical charts was lung cancer in 99% of patients aged < 55 while 1% died of other causes. With increasing age the cancer-related mortality dropped to 87% in the oldest age group. There was a higher occurrence of advanced stages in younger patients (84% with stage III-IV in the youngest age group compared with 58% in patients ≥ 75 years). Of the 952 patients for whom information concerning relapse was available, 753 (79%) had a confirmed relapse. The incidence of relapse was highest in the youngest age group (83%) and decreased with increasing age to 69% in patients aged ≥ 75 years. Mean radiation dose and range was 58 (50–68) Gy for patients < 55 years, 59 (50–70) Gy for patients 55–64 years, 59 (50–74) Gy for patients 65–74 years and 59 (50–70) Gy for patients aged ≥ 75 years. Some patients had surgery and/or chemotherapy in addition to radiotherapy. Both surgery and chemotherapy was more often given to the younger patients and its use decreased with increasing age. A summary of patient characteristics, given treatment, occurrence of relapse and cause of death is provided in .
Table I. Patient characteristics, treatment and relapse.
Age and survival
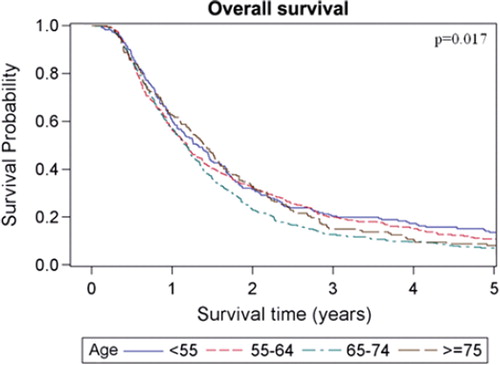
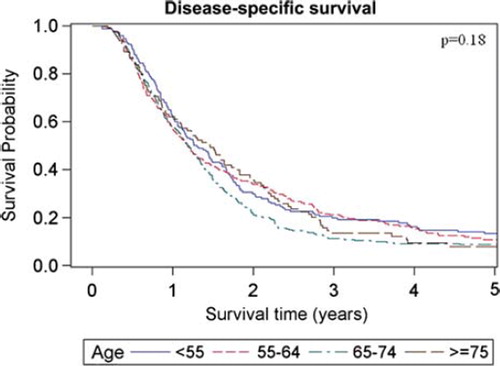
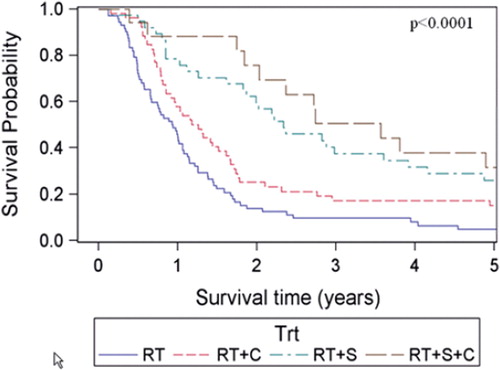
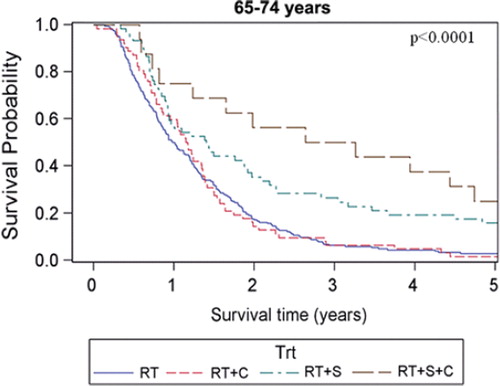
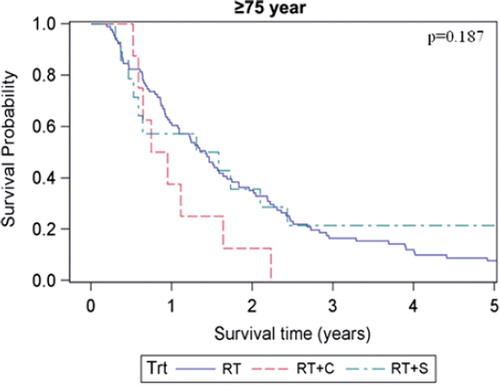
The estimated median overall survival of all patients was 14.7 months (95% CI 13.8–15.5 months) and the five-year survival rate was estimated to 9.5%. Overall and disease-specific survival for patients in the four different age groups are shown in and . For overall survival there was a statistically significant difference among age groups (p = 0.017, log-rank test), whereas no difference could be shown for disease-specific survival (p = 0.18, log-rank test). When comparing patients with early (stage I-II) versus advanced (stage III-IV) staged cancer there was only a significant overall survival difference in the age group of patients < 55 years, (p = 0.015, log-rank test). When comparing patients receiving chemotherapy (induction and/or concomitant, see ) with those who did not, the survival benefit of chemotherapy was most pronounced in the youngest age group and decreased with increasing age. For the patients aged ≥ 75, the group receiving chemotherapy tended to have shorter median overall survival (10.2 months compared with 17.1 months in the group that received only radiotherapy), However, only eight of 158 patients in this age group were given chemotherapy. For patients subjected to surgery and postoperative radiotherapy there was a major survival advantage for the patients in the three younger age groups who had received this treatment compared with radiotherapy alone. However, for patients aged ≥ 75 the median overall survival was roughly the same for both the operated and non-operated patients (17.3 months and 17.1 months, respectively) whereas the five-year overall survival was 21.4% in the operated patients and 7.7% in the non-operated patients. It should be noted, however, that only 14 of 113 patients in this age group received surgery. For patients subjected to surgery and postoperative radiotherapy with the addition of chemotherapy (induction and/or concomitant), the survival advantage with respect to patients receiving radiotherapy alone was markedly increased compared to when receiving either surgery or chemotherapy in addition to radiotherapy in the three youngest age groups. For patients aged ≥ 75, there were too few patients receiving trimodal treatment (n = 2) for a meaningful statistical analysis. The overall survival for the different age groups in patients receiving different combinations of treatment is shown in . Estimated median survival for different subgroups is shown in (overall survival) and (disease-specific survival).
Table IIa. Estimated overall survival for different subgroups.
Table IIb. Estimated disease-specific survival for different subgroups.
The univariate Cox-analyses showed that the variables age (65–74 years), WHO performance status, histopathology (SCC), stage, surgery, and first and second line chemotherapy were statistically significantly associated with survival, while gender, time period and smoking status were not (). High WHO performance status grade, squamous cell carcinoma and advanced stage were associated with poorer survival (p < 0.0001, p = 0.0074 and p < 0.0001, respectively) whereas the addition of surgery or chemotherapy to radiotherapy were associated with better survival (p < 0.0001 and p < 0.0001, respectively). Also, patients who received chemotherapy in a second line setting had a better overall survival than those who did not (p = 0.028). When using patients aged < 55 years as a reference in the univariate analysis we found that they had significantly better overall survival than patients aged between 65–74 years (p = 0.0024). In a multivariate Cox-analysis (), all the above mentioned variables, except time period and smoking status, were included. The significant relationship with survival was retained for age 65–74 years (p = 0.0097), WHO performance status (p < 0.0001) stage (p = 0.0032), surgery (p < 0.0001), first-line (p = 0.022) and second-line chemotherapy (p = 0.0009). In addition, age 55–64 years was also significantly associated with poorer survival in the multivariate analysis (p = 0.035). However, patients aged ≥75 years did not have a significantly poorer survival than patients <55 years.
Table III. Univariate Cox analyses of overall survival.
Table IV. Multivariate Cox analysis of overall survival.
Discussion
To our knowledge, the present report describes the largest clinical study analysing the relationship between outcome of treatment of non-small cell lung cancer and age at diagnosis. We found that patients younger than 55 years treated with curatively intended radiotherapy for NSCLC had an overall survival which in a multivariate Cox-model was shown to be statistically significantly better than patients aged between 55–64 and 65–74 years, whereas no significant difference in overall survival could be shown when comparing the youngest and the oldest age group. The addition of surgery or chemotherapy for patients 75 years or older does not seem to give any survival benefit and may instead be detrimental for some older patients.
Since almost all patients that have been localised were included and no intentional selection has been performed the study population literally reflects reality, which is one of the greatest strengths of the current study protocol. On the other hand, this can also be regarded as a drawback as there were no proper inclusion criteria or common treatment protocol, which makes the cohort rather heterogeneous and the results harder to interpret. One direct consequence of this was that patients in stages I-II and IV, who primarily are not treated with radiotherapy, were included in the study. The included patients in stages I-II received radiotherapy either postoperatively after non-radical surgery or because they had for some reason been considered inoperable. The included patients in stage IV were given curative treatment despite radiological signs of possible dissemination at the start of treatment which were later confirmed to be distant metastases. However, apart from minor differences in chemotherapeutic agents being used, there were no major differences in the treatment protocols being used at the different hospitals as the radiation treatment protocols followed the Swedish national guidelines eligible at the time. The national guidelines for radiotherapy has not changed much in recent years, as compared to the time period of the present study, except for an escalation of the radiation dose.
There are other limitations with the present study that are related to its retrospective nature and historical context. The TNM classification was based on the available imaging techniques during the different time intervals which may have changed over time due to the development of more sensitive staging techniques such as magnetic resonance imaging (MRI), four-dimensional computed tomography (4D-CT), positron emission tomography (PET)/CT [Citation7]. Today, higher doses of radiotherapy can be delivered due to these imaging innovations [Citation8]. Moreover, the histopathological/cytological specimens were not reviewed for a second opinion and, the patients were not treated or followed according to a standardised protocol. Further, since the majority of patients throughout the study period were never autopsied, the cause of death might not be thoroughly investigated resulting in difficulties analysing disease-specific survival, especially so among the elderly patient population. Also, there were missing values for some of the explanatory variables, which may lead to a selection bias.
During the past years, patients with NSCLC have often been given similar treatment, regardless of age and other prognostic information. The issue concerning age has further been rather confusing since some studies have reported that younger patients have a worse prognosis than older patients [Citation9–11] whereas other reports show similar overall survival rates [Citation12–14] and further a number of studies have shown better overall survival rates in younger patients [Citation15–17]. In the present study our aim was to clarify the difference of the clinical outcome in patients related to age at the time of diagnosis. The cohort was divided into four groups; <55, 55–64, 65–74 and ≥75 years. From our point of view, the above described age groups are of importance since we tend to define patients as “young” if <55 years of age and if a patient is ≥75 years of age, we tend to define this patient as “old”. In Sweden the age of retirement is 65 years and we decided to split the middle-aged group into a “young middle-aged group”; 55–64 and an “old middle-aged group”; 65–74 years. In terms of patient characteristics we found several differences between these groups. Among the youngest patients there was a predominance of female gender which steadily declined with increasing age. Adenocarcinomas were most prevalent among the youngest patients whereas squamous cell carcinomas increased with rising age, while the proportion of other histologies remained relatively constant with increasing age. This is in accordance with other reports which show a higher proportion of women and adenocarcinomas in the younger patient population affected with NSCLC [Citation18,Citation19]. We found that thoracic pain seemed to decrease with increasing age, whereas the opposite was observed for haemoptysis. This is in line with a study by Nugent et al. in which thoracic pain was found to be significantly more common in younger (<45 years) than in older patients (>80 years). Moreover, haemoptysis was more common in the older age group but the difference was not statistically significant [Citation17].
We also found that patients aged <55 years had the highest five-year overall survival rate. This was found in a multivariate analysis where possible confounders like performance status, stage and different treatments given were taken into account. However, the relationship between advanced age and survival was not consistent since patients aged ≥75 years had the longest median survival time among the age groups (17.1 months), whereas the five-year overall survival rate was only 8.1% as compared with 13.6% for patients aged <55 years. These contradictory results may perhaps be attributed to a less aggressive treatment approach among the elderly resulting in a lower initial mortality. Furthermore, more indolent tumour biology in elderly patients could contribute to the lower initial mortality in this age group. Moreover, patients ≥75 years seem to have a higher risk of death by other causes than lung cancer five years after the day of diagnosis. The subject is controversial. There are reports in accordance with the present study suggesting better prognosis of survival for younger patients [Citation9–11] as well as reports finding no such difference or even a worse prognosis in younger patients [Citation12,Citation15,Citation16]. Since the cause of death was lung cancer in 93% of the patients, the disease-specific survival was as expected similar to the overall survival. However, when comparing the impact of stage on survival for patients in the different age groups, we found that those with stages I-II had a significant survival advantage compared with patients with clinical stages III-IV for patients aged <55 years, whereas for all the other age groups there was no significant survival benefit. Thus, it seems that clinical stage has the most prognostic importance in patients younger than 55 years, while its prognostic significance in elder patients is less certain. However, among the patients in stages I-II who have been treated with radiotherapy, there are those who were inoperable for medical reasons and those who had underwent non-radical surgery prior to radiotherapy, thus reducing the prognostic benefit of a lower stage. This selection bias also makes comparison of the results with those obtained with surgical treatment (pneumectomy, lobar resection) of stage I-II disease misleading since patients able to tolerate surgery are by definition healthier [Citation20]. When investigating patients who received surgery and postoperative radiotherapy we found a major survival benefit for operated patients aged younger than 75 years. In contrast, patients aged ≥75 years surgical intervention did not give a median overall survival benefit whereas it did give a higher five-year overall survival. These results may seem contradictory but since only 14 operated patients aged ≥75 years were included in this study it is not possible to draw any conclusions about the efficacy of surgery in elderly patients from these results alone. In a study by Noordijk et al. the authors found no difference in the outcome of elderly patients treated with radiotherapy alone or surgery which is in line with the present study [Citation21]. Regarding patients receiving chemotherapy in a neoadjuvant and/or concomitant fashion, there was a survival benefit for patients aged <75 years, most pronounced in the youngest age group.
In conclusion, this study indicates that younger patients, despite having a more aggressive disease and higher clinical stage at presentation, have the best prognosis of overall survival. Furthermore, the present study suggests that older patients may not benefit from a more aggressive treatment approach involving chemotherapy or surgery in addition to radiotherapy, which should be taken into consideration when making treatment decisions for this group of patients. Also, with the addition of more modern staging techniques, such as PET/CT, it will be easier to accurately select patients suitable for curatively intended radiotherapy by excluding those with stage IV disease. The results obtained in this study should therefore be confirmed by future prospective clinical trials involving radiation treatment of patients with non-small cell lung cancer.
Acknowledgements
The authors would like to thank Stiftelsen Gävle Cancerfond for generously providing funds for this project. Also, the authors would like to thank all those who have helped with the gathering of patients and their medical charts, especially the following persons: Ola Brodin, Claes Mercke, Jan-Erik Westlin, Enyat Mavadati, Andrzej Piwowar, Ingemar Sandin, Daniel Brattström, Shirin Mavadati, Sutharsan Suntharalingam, Peter Ericsson and Mats Fagerlind. None of the authors in this study has any financial or personal relationships with other people or organisations that could have inappropriately influenced their work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
- Caputi M, Russo G, Esposito V, Mancini A, Giordano A. Role of cell-cycle regulators in lung cancer. J Cell Physiol 2005;205:319–27.
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26.
- Atagi S, Kawahara M, Tamura T, Noda K, Watanabe K, Yokoyama A, . Standard thoracic radiotherapy with or without concurrent daily low-dose carboplatin in elderly patients with locally advanced non-small cell lung cancer: A phase III trial of the Japan Clinical Oncology Group (JCOG9812). Jpn J Clin Oncol 2005;35:195–201.
- Gridelli C, Langer C, Maione P, Rossi A, Schild SE. Lung cancer in the elderly. J Clin Oncol 2007;25:1898–907.
- Tai BC, Machin D, White I, Gebski V. Competing risks analysis of patients with osteosarcoma: A comparison of four different approaches. Stat Med 2001;20:661–84.
- Partridge M, Yamamoto T, Grau C, Hoyer M, Muren LP. Imaging of normal lung, liver and parotid gland function for radiotherapy. Acta Oncol 2010;49:997–1011.
- Moller DS, Khalil AA, Knap MM, Muren LP, Hoffmann L. A planning study of radiotherapy dose escalation of PET-active tumour volumes in non-small cell lung cancer patients. Acta Oncol 2011;50:883–8.
- Bryant AS, Cerfolio RJ. Differences in outcomes between younger and older patients with non-small cell lung cancer. Ann Thorac Surg 2008;85:1735–9; discussion 1739.
- Bourke W, Milstein D, Giura R, Donghi M, Luisetti M, Rubin AH, . Lung cancer in young adults. Chest 1992;102:1723–9.
- Antkowiak JG, Regal AM, Takita H. Bronchogenic carcinoma in patients under age 40. Ann Thorac Surg 1989; 47:391–3.
- Bernet F, Brodbeck R, Guenin MO, Schupfer G, Habicht JM, Stulz PM, . Age does not influence early and late tumor-related outcome for bronchogenic carcinoma. Ann Thorac Surg 2000;69:913–8.
- Gadgeel SM, Severson RK, Kau Y, Graff J, Weiss LK, Kalemkerian GP. Impact of race in lung cancer: analysis of temporal trends from a surveillance, epidemiology, and end results database. Chest 2001;120:55–63.
- Green LS, Fortoul TI, Ponciano G, Robles C, Rivero O. Bronchogenic cancer in patients under 40 years old. The experience of a Latin American country. Chest 1993; 104:1477–81.
- Serrano-Olvera A, Gerson R. [Age associated survival rate in non small cell lung cancer]. Gac Med Mex 2009;145: 27–35.
- Kuo CW, Chen YM, Chao JY, Tsai CM, Perng RP. Non-small cell lung cancer in very young and very old patients. Chest 2000;117:354–7.
- Nugent WC, Edney MT, Hammerness PG, Dain BJ, Maurer LH, Rigas JR. Non-small cell lung cancer at the extremes of age: Impact on diagnosis and treatment. Ann Thorac Surg 1997;63:193–7.
- Blanco M, Garcia-Fontan E, Rivo JE, Repaaz JR, Obeso GA, Canizares MA. Bronchogenic carcinoma in patients under 50 years old. Clin Transl Oncol 2009;11:322–5.
- Hsu LH, Chu NM, Liu CC, Tsai SY, You DL, Ko JS, . Sex-associated differences in non-small cell lung cancer in the new era: Is gender an independent prognostic factor? Lung Cancer 2009;66:262–7.
- Robinson CG, Bradley JD. The treatment of early-stage disease. Semin Radiat Oncol 2010;20:178–85.
- Noordijk EM, vd Poest Clement E, Hermans J, Wever AM, Leer JW. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988;13:83–9.