Abstract
Background. We aimed to derive three-dimensional volume-based (V3D) response criteria that approximate those based on Response Evaluation Criteria in Solid Tumours (RECIST) in patients with brain metastases (BM) treated with salvage stereotactic radiosurgery (SRS). Material and methods. Seventy patients with 178 BM were treated with SRS. Each BM was characterised at baseline and at each follow-up MRI according to its widest diameter and V3D using ITK-SNAP image segmentation software. Results. The median tumour diameter was 1.2 cm (range, 0.2–4.5 cm) and V3D was 0.73 cm3 (range, 0.01–22.7 cm3). The V3D percent changes that best matched RECIST response criteria were: an increase of ≥71.5% for progressive disease, a ≥58.5% decrease for partial response and a <58.5% decrease or increase of <71.5% for stable disease (k =0.85). A baseline diameter >3.0 cm (p =0.006) and a V3D >6.0 cm3 (p =0.043) predicted for local failure, and a baseline cumulative V3D of >3.0 cm3 (p =0.02) was adversely prognostic for survival. Conclusions. We define 3D volume specific criteria to base response upon for brain metastases treated with salvage SRS. Tumours with a V3D of greater than 6 cm3 are at a higher risk of local failure.
Response criteria for tumours in general have been traditionally based on changes in linear dimensions and not true three-dimensional (3D) volume based assessments [Citation1]. Specific to the reported clinical trials literature for brain metastases, there have been no consistent criteria for response determination, and some have used Response Evaluation Criteria In Solid Tumours (RECIST) guidelines [Citation1–3], some have used World Health Organization (WHO) based criteria [Citation4,Citation5] and others have applied locally derived criteria [Citation6]. This heterogeneity in response classification is hazardous when comparing outcomes, and RECIST was in fact developed to overcome this barrier [Citation1]. However, when considering how to categorise tumour response in a cohort of patients with brain metastases treated with stereotactic radiosurgery (SRS), we found the current gold standard RECIST [Citation1] to be lacking.
The limitations of RECIST [Citation1], and any linear dimension based criteria for that matter, include generalising the complexity of tumour geometry to a linear dimension, the difficulty in estimating the maximum tumour diameter for irregular or confluent lesions [Citation7], discrepancies in scan planes and patient positioning can result in erroneous measurements, the number of tumours within an organ are limited to no more than five, the smallest tumour permitted to be tracked is 1 cm in maximal diameter [Citation1], and in cases of multiple tumours the dimensions of the individual tumours are summed to yield an overall response classification [Citation1] rather than considering each tumour independently.
With modern technology allowing for segmentation of individual tumours on diagnostic magnetic resonance (MR) images, we are now able to obtain accurate 3D volume measurements of individual lesions with relative ease. 3D volumes will undoubtedly replace the more crude and imprecise linear measurement approach, and should yield more accurate subsequent response assessments. This is particularly relevant for brain metastases, as we often treat multiple tumours, small sub-centimetre tumours, and each treated metastases should be considered biologically independent given that growth of any one lesion has a clinical impact on the patient's treatment course. However, one barrier to adopting 3D volume-based measurements has been the lack of formally recognised percent volume change criteria to base response upon. The aim of this study was to derive 3D volume-based criteria for response that best approximate RECIST classifications, and specific to brain metastases treated with SRS.
Material and methods
All patients with brain metastases treated with Gamma Knife SRS [Citation8] between December 2005 and May 2008 were retrospectively reviewed. One hundred and nineteen patients with 287 brain metastases were identified. Patients were excluded if metastases were located within the brainstem, had been treated with the intent of boost SRS (pre- or post-WBRT within eight weeks of SRS) or if no follow-up imaging was performed. The final cohort consisted of 70 patients with 178 treated metastases ().
Table I. Baseline patient and tumour characteristics (n =70 patients, 178 metastases).
Our first task was to classify the response of each treated brain metastases according to the RECIST [Citation1] classifications () of partial response (PR, > 30% decrease in maximum diameter), stable disease (SD <30% decrease and <20% increase in maximum diameter), and progressive disease (PD > 20% increase in maximum diameter). Complete response (CR) was defined as the complete disappearance of the tumour.
Table II. RECIST response criteria according to Therasse et al. [Citation1] and the derived percent volume changes that co-relate with RECIST for Vc and V3D based on the present analysis.
The primary objective of this study was to then derive those 3D volume (V3D), and calculated sphere volume (Vc), percent volume change criteria that would best approximate the response classifications defined by RECIST. Importantly, we determined response for each lesion independently, and did not sum diameters or volumes nor consider grouping targets into a single metric when multiple lesions were treated in the same patient. New metastases were not considered unless they were treated with SRS and followed, as our intent was focussed on local control of only those radiosurgerised metastases.
In our cohort, 95% of patients had been treated with salvage intent in patients previously irradiated with whole brain radiotherapy (WBRT). Therefore, our secondary objective was to determine those predictive and prognostic factors in the salvage SRS setting that can help identify patients best suited for this therapy, given that these factors are not well described in the literature.
Treatment
All patients were treated with the Gamma Knife 4C (Elekta Instruments, Atlanta, GA, USA) unit [Citation8]. Briefly, the treatment process consists of applying a Leksell invasive stereotactic head frame for rigid head fixation on the day of SRS. Then T1-post gadolinium MRI axial images and axial T2-fluid attenuation inverse recovery images were obtained with the frame in place for subsequent treatment planning. All patients were treated with single fraction SRS. The dose guidelines were based on RTOG criteria [Citation9]; however, modified according to the calculated volume of a sphere. Our guidelines suggest doses of 24 Gy, 21–24 Gy, 18 Gy, and 15 Gy or less for tumour volumes of <4 cm3, 4–8 cm3, 8–14 cm3, and 14–30 cm3, respectively, and typically prescribed to the 50% isodose. The doses in this study ranged from 12–24 Gy (median, 24 Gy), and the prescription isodose ranged from 40–95% (median, 50%). Lower doses, and a higher prescription isodose, than those recommended were generally prescribed for lesions located near critical structures or for very large tumours.
Follow-up and treatment response
Patients were followed with gadolinium contrast-enhanced brain MRIs every 2–5 months (median, 3 months) following SRS. Using ITK-SNAP 1.8.0 image segmentation software [Citation10] we recorded individual tumour measurements that included the true 3D segmented volume (V3D), and the maximal x, y and z linear dimensions, at baseline (day of SRS) and at each subsequent follow-up image. All image segmentations were performed by a single investigator (KK) for consistency. We used the maximum linear diameter in any plane for subsequent RECIST response evaluation [Citation1], and to determine the Vc according to Vc =4/3πr3 (r is the radius and determined by dividing the maximum diameter by 2). We first determined the response of each treated metastases according to RECIST, and if PD was determined (or if further intervention with surgery or whole brain radiotherapy occurred), then the tumour was censored at that time.
Statistical analysis
The primary objective of this study was to derive the V3D and Vc percent volume changes for PD, SD, PR and CR, that would best approximate tumour response as classified first by RECIST criteria. The weighted Kappa co-efficient was used to evaluate the agreement using RECIST as the reference standard.
Descriptive statistics were reported with median and range for continuous variables, and frequencies and proportions for categorical variables. Association was analysed between the predictors and clinic outcomes such as local control and overall survival. Overall survival and time to PD were calculated using the Kaplan-Meier method. The Cox-proportional hazard regression model was applied to estimate the hazard ratio (HR) and 95% confidence interval (CI).
Factors analysed for predictors of local tumour control included primary cancer type, metastasis location (supratentorial, infratentorial), prescribed dose (12–15 Gy, 18–21 Gy, 24 Gy), baseline metastasis diameter (<1.0 cm, 1.0–3.0 cm, >3.0 cm) and baseline segmented volume (>6.0 cm3 vs. ≤6.0 cm3). Factors analysed as potential predictors of overall survival included primary cancer type, patient age (<60 years, ≥60 years), Karnofsky Performance Status (KPS) (<70, 70–80, 90–100), Recursive Partitioning Analysis [Citation11] (RPA) category, Graded Prognostic Index [Citation12] (GPA) score (≤2.5, >2.5), number of treated metastases (1–4, ≥4), SRS intent (salvage or primary), baseline metastasis diameter (<3.0 cm, ≥3.0 cm) and baseline cumulative total segmented volume (<3.0 cm3, 3.0–6.0 cm3, 6.0–10.0 cm3, and >10.0 cm3). Based on those significant factors (p ≤0.05) identified from the univariate analyses, a multivariate analysis was performed. Two-sided tests were applied. Results were considered significant if the p-value was ≤0.05. Statistical analyses were performed using Version 9.2 of the SAS system and user's Guide (SAS Institute, Cary, NC, USA).
Results
Patient, metastasis, and treatment characteristics
Patient characteristics are reported in . Thirty-seven patients (51%) were treated for a solitary brain metastasis, otherwise the number of brain metastases treated ranged from 2 to 18. The median maximum linear diameter was 1.2 cm (range, 0.2–4.5 cm), V3D was 0.73 cm3 (range, 0.01–22.7 cm3) and Vc was 0.7 cm3 (range, 0.004–47.1 cm3). The most common indication for SRS was to salvage metastases in patients treated with previous WBRT (95%). The median follow-up post-SRS was 18.3 months.
Target response and concordance of Vs, Vc and RECIST
Based on RECIST criteria [Citation1], we classified the response of each tumour treated at 3, 6, 9, 12, 18 and 24 months post-SRS with respect to CR, PR, SD and PD (). We then derived the optimal percent volume changes for both V3D and Vc that would yield the highest concordance with those RECIST response classifications determined at each follow-up time-point. We clearly observe in the consistency in the rates of CR, PR, SD and PD at each follow-up time point according to RECIST, V3D, and Vc. Based on the last follow-up assessment, the weighted kappa correlation statistic (k) for V3D and RECIST was 0.85 (p <0.0001), and for Vc and RECIST was 0.95 (p <0.0001). Furthermore, consistency was observed when considering the median time to progression according to RECIST, V3D and Vc (14, 13, and 13 months, respectively), and according to the 1 and 2 year accumulative PD rates (0.41, 0.46, and 0.45 and 0.74, 0.76 and 0.76, respectively). Those experimentally derived percent volume change criteria for V3D and Vc that yielded this high level of agreement to RECIST are summarised in .
Table III. Target response rates according to RECIST, Vc and V3D at each specified follow-up.
Predictors and prognostic factors
We performed a univariate analyses to determine factors predictive of local control () according to each criteria. For maximum linear diameter tumour measurements RECIST criteria apply, and we observe metastases with a baseline diameter >3.0 cm to be at higher risk of PD (). According to V3D tumour measurements, we observe a significant relationship between those tumours with a V3D of ≥6.0 cm3 (p =0.043, , ) and a greater risk of PD. At the time of our analysis, 26 patients had died, the median survival time was 15.3 months (95% CI 8.3–20.7), and the 1- and 2-year survival probabilities were 0.54 and 0.10, respectively. Univariate analyses were performed to assess prognostic factors (), and a baseline cumulative V3D of <3.0 cm3 (p =0.02) was the only factor associated with greater overall survival ().
Figure 1. Intracranial progressive disease rates according to baseline segmented contrast volume (V3D) >6 cm3 vs. ≤6 cm3.
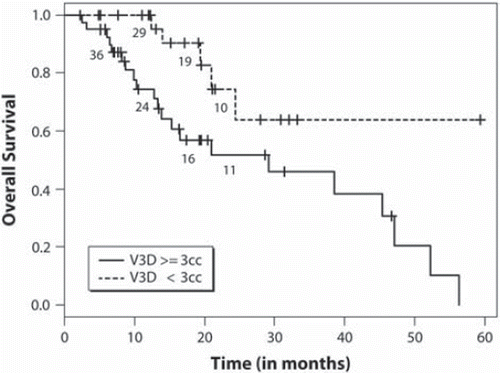
Figure 2. Overall survival distribution based on cumulative tumour baseline segmented contrast volume (V3D) ≥3 cm3 vs. <3 cm3.
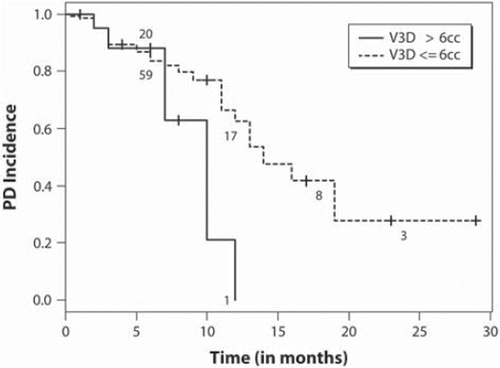
Table IV. Predictors of progressive local disease.
Table V. Prognostic factors for overall survival.
Discussion
The major challenge in adopting 3D volume based measurements to determine tumour response has been the lack of formalised response criteria. Several investigators have reported concordance in response categorisation when comparing uni- and bi-dimensional measurements; however, discordance when compared to 3D volumetric assessments [Citation13,Citation14]. The discordance is likely based on the incorrect assumption that percent volume changes extrapolated from RECIST to determine PD, SD, and PR based on a sphere volume would be valid for true 3D volume assessments. Our aim was to derive the percent volume change criteria that apply to 3D segmented tumour volume measurements, for radiosurgerised brain metastases, indexed to RECIST response criteria as the reference standard.
We first report RECIST based CR, PR, SD and PD responses for brain metastases treated with SRS. We then derived the optimal percent volume change criteria for Vc and V3D measurements that best matched those RECIST-based response classifications at each follow-up time point. The consistencies in the response rates at each follow-up assessment based on RECIST, Vc and V3D are clearly observed in , and compare well to the published SRS literature [Citation15]. We then summarise those derived Vc and V3D percent volume change criteria for each response classification (CR, PR, SD and PD) in that yielded this high level of agreement.
We first report an almost perfect concordance in Vc response classifications with RECIST (k =0.95). We don't expect to see perfect agreement, i.e. a k =1, as Vc is not a perfect linear function of RECIST, and the k value here is to evaluate the agreement between two response criteria that are based on corresponding percentage changes. We also observe that the percent volume changes according to Vc were not the same as those we derived according to V3D measurements (). This reflects that true segmented 3D volume measurements (V3D) are independent of those simply calculated. Our analysis supports an increase of at least 71.5% in V3D to classify PD, a reduction in V3D by at least 58.5% to classify a PR and SD classified by a reduction of less than 58.5% and an increase no more than 71.5% in V3D (with RECIST as the reference standard). Based on these derived response criteria for V3D we observe a high level of agreement with RECIST as the k =0.85.
Our secondary endpoints were to determine prognostic and predictive factors specific to salvage SRS given that 95% of our patients had been treated with prior WBRT. There is limited literature specific to this population. We observed using linear dimensions and RECIST criteria that tumours greater than 3 cm are at greater risk of PD. The relationship to increasing tumour diameter and increasing risk of PD has been well described and; therefore, our results consistent [Citation16–20]. Based on V3D, we determined a volume of 6 cm3 as a predictive factor for PD, and this outcome is valuable given the lack of true 3D volume analysis in the literature post-SRS. A volume of 6 cm3 roughly translates to a maximum linear diameter of 2 cm. Therefore, we are again consistent with the literature [Citation20,Citation21]; however, specific to V3D. With respect to cumulative volume, we did not observe any relationship with individual tumour local control. This implies that each tumour should be considered independently rather than a single measure based on a sum of individual tumour dimensions. With respect to survival, we observed a cumulative V3D of > 3 cm3 as a significant prognostic factor. We did not observe those traditional factors associated with survival and brain metastases as prognostic which include RPA class and GPA score. This may be a function of the patient population under study, as these well known prognostic factors were determined for patients treated upfront for brain metastases with radiation and not in patients treated with SRS as salvage post-WBRT. Within the limited literature reporting outcomes for this patient cohort, similar observations have been made [Citation21–23].
Although the population under study is the one of the largest to be analysed with the intent of determining V3D response thresholds, these criteria need to be tested in a larger population and validated. However, the statistical significance is strong and compelling such that use of these criteria is justified. Other limitations include the population under study, where 95% of patients had previous WBRT and treated with SRS as salvage. We were unable to judge with certainty whether tumours were treated for progression despite WBRT or due to new tumours developing in the previously radiated brain. This was due to the predominant use of CT at the time of WBRT, lack of imaging-based follow-up post-initial WBRT, and MR imaging only performed at the time of referral for salvage SRS. However, there are no compelling data to suggest that there is a differential response according to these two clinical scenarios. We also did not compare bi-dimensional measurements as per WHO response criteria [Citation5]. Given that other investigators have shown concordance between determined response categories when using either RECIST or WHO [Citation13,Citation14], we did not feel this to be necessary and limited our analysis to RECIST. With respect to possibility of radiation necrosis resulting in a sufficient increase in tumour dimension to score PD as opposed to true PD, this is a limitation of the study. We censored treated tumours once the criteria had been met for PD, and did not bias the analysis by then retrospectively scoring those PD cases that could have been necrosis according to further imaging analysis. There are major challenges in diagnosing radiation necrosis from true PD and a definitive diagnosis can only be made upon surgical resection and pathological evaluation. Moreover, radiation necrosis is not common (∼2% [Citation6]), and we felt that it was not a factor in our patient population upon review of the imaging. Similarly, there may be some treated lesions that transiently increase in size and then stabilise or regress analogous to pseudoprogression in glioma [Citation24]. This potential treatment effect is not well described and in our analysis occurred in only 2% of cases (data not shown). Lastly, whether these criteria are generalisable to other treatments for brain metastases (studies evaluating the response of brain metastases to chemotherapy, whole brain radiotherapy, ultrasound), or to any tumour for that matter is unknown and a subject of further study.
In conclusion, we define 3D tumour volume response criteria in a population of patients treated with SRS for brain metastases based on RECIST criteria as the reference standard. These criteria are a step forward in formalising 3D volume based tumour measurements as a future standard to replace linear-based measurements. We also observe that in the salvage setting, individual metastases with a baseline 3D volume of greater than 6 cm3 are at higher risk of local failure, and a baseline cumulative 3D tumour volume of >3.0 cm3 (p =0.020) was adversely prognostic for survival.
Declaration of interest: None of the authors have any actual or potential conflicts of interest.
References
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Tsimberidou AM, Letourneau K, Wen S, Wheler J, Hong D, Naing A, . Phase I clinical trial outcomes in 93 patients with brain metastases: The MD Anderson Cancer Center Experience. Clin Cancer Res 2011;17:4110–8.
- Rodrigues G, Eppinga W, Lagerwaard F, de Haan P, Haasbeek C, Perera F, . A pooled analysis of arc-based image-guided simultaneous integrated boost radiation therapy for oligometastatic brain metastases. Radiother Oncol 2012;102:180–6.
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol 2009;10:1037–44.
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–14.
- Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg 2006;104:907–12.
- Hopper KD, Kasales CJ, Van Slyke MA, Schwartz TA, TenHave TR, Jozefiak JA. Analysis of interobserver and intraobserver variability in CT tumor measurements. Ajr 1996;167:851–4.
- Sahgal A, Ma L, Chang E, Shiu A, Larson DA, Laperriere N, . Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat 2009;8:271–80.
- Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, . Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 2000;47:291–8.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, . User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006;31:1116–28.
- Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, . Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997; 37:745–51.
- Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510–4.
- Prasad SR, Jhaveri KS, Saini S, Hahn PF, Halpern EF, Sumner JE. CT tumor measurement for therapeutic response assessment: Comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology 2002;225:416–9.
- Tran LN, Brown MS, Goldin JG, Yan X, Pais RC, McNitt-Gray MF, . Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest computed tomography. Acad Radio 2004;11:1355–60.
- Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer Epub 2011 Sep 1.
- Chang EL, Hassenbusch SJ, 3rd, Shiu AS, Lang FF, Allen PK, Sawaya R, . The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery 2003;53:272–80; discussion 80–1.
- Selek U, Chang EL, Hassenbusch SJ, 3rd, Shiu AS, Lang FF, Allen P, . Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys 2004;59:1097–106.
- Seung SK, Sneed PK, McDermott MW, Shu HK, Leong SP, Chang S, . Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am 1998;4:103–9.
- Yu C, Chen JC, Apuzzo ML, O'Day S, Giannotta SL, Weber JS, . Metastatic melanoma to the brain: Prognostic factors after gamma knife radiosurgery. Int J Radiat Oncol Biol Phys 2002;52:1277–87.
- Hoffman R, Sneed PK, McDermott MW, Chang S, Lamborn KR, Park E, . Radiosurgery for brain metastases from primary lung carcinoma. Cancer J (Sudbury, Mass.) 2001;7:121–31.
- Chao ST, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Neyman G, . Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer 2008;113:2198–204.
- Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL, . Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys Epub 2011 Nov 11.
- Maranzano E, Trippa F, Casale M, Costantini S, Anselmo P, Carletti S, . Reirradiation of brain metastases with radiosurgery. Radiother Oncol Epub 2011 Aug 29.
- Sanghera P, Perry J, Sahgal A, Symons S, Aviv R, Morrison M, . Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 2010;37:36–42.
