Abstract
Background: Current guidelines are inconclusive regarding intravenous (IV) iron for treatment of chemotherapy-induced anaemia (CIA). Material and methods: Systematic review and meta-analysis of randomised controlled trials comparing IV iron with no iron or oral iron for treatment of chemotherapy induced anaemia (CIA). Primary outcomes: haematopoietic response and red blood cell (RBC) transfusion requirements. For dichotomous data, relative risks (RR) with 95% confidence intervals (CIs) were estimated and pooled. For continuous data, weighted mean differences were calculated. Results: Eleven trials included 1681 patients, the majority examining the addition of IV iron to erythropoiesis stimulating agents (ESA) (1562 patients, 92.9%). IV iron significantly increased haematopoietic response rate [RR 1.28 (95% CI 1.125–1.45), seven trials with ESA] and decreased the rate of blood transfusions both in trials with ESA [RR 0.76 (95% CI 0.61–0.95), seven trials] and without ESA [RR 0.52 (95% CI 0.34–0.80)]. The increase in haematopoietic response rate correlated with total IV iron dose, regardless of baseline iron status. Mortality and safety profile was comparable between groups. Conclusions: IV iron added to ESA results in an increase in haematopoietic response and reduction in the need for RBC transfusions, with no difference in mortality or adverse events.
Anaemia is an almost universal complication in cancer patients and an important contributor to morbidity of malignancy [Citation1]. A European prospective survey, found that the prevalence of anaemia in cancer patients was 39.3% at enrolment, and increased to 67% during the observation period [Citation1]. The pathophysiology of anaemia in cancer is multifactorial, but in most cases results from anaemia of chronic disease [Citation2]. Chemotherapy further exacerbates the anaemia due to impaired erythropoiesis [Citation3].
Erythropoiesis stimulating agents (ESAs) have been shown by several clinical trials to correct chemotherapy-induced anaemia (CIA) and reduce the need for transfusions [Citation4,Citation5] and may currently be considered for specific settings of cancer patients receiving chemotherapy [Citation6], mainly with palliative intent in order to reduce the need for transfusions [Citation7,Citation8]. However, only 40–70% of patients with cancer achieve a haematological response with ESA [Citation9].
One of the most important causes of ESA unresponsiveness is functional iron deficiency, characterised by iron restricted erythropoiesis, meaning, a failure to provide iron to the erythroid marrow despite sufficient iron stores [Citation10]. Furthermore, patients who are not iron deficient may develop iron deficiency on ESA therapy [Citation11]. To avoid it, concomitant iron treatment was suggested [Citation7–9]. Since oral iron is poorly absorbed in anaemia of chronic disease due to increase in inflammatory cytokines and hepcidin [Citation10], it has not been thoroughly studied in clinical trials in the setting of cancer-related anaemia. On the other hand, intravenous (IV) iron may have the potential to overcome iron restricted erythropoiesis in this population [Citation12,Citation13].
Recommendations of the guidelines regarding IV iron supplementation are inconsistent. The 2010 ASH/ASCO guidelines do not consider the use of IV iron as a standard of care [Citation7]. The EORTC guidelines cite improved response to ESA with IV iron (but not oral) but indicate the need to define optimal dose and schedule [Citation9], the 2010 ESMO guidelines suggest iron supplementation for iron deficient patients [Citation6], and the NCCN guidelines consider iron supplementation, especially IV, for functional iron deficiency, if ferritin level is less than 800 ng/ml and transferrin saturation (TSAT) is less than 20% [Citation8].
Since these guidelines were published, additional randomised controlled trials assessing the use of IV iron with ESA for the treatment of CIA were published, with conflicting results [Citation14,Citation15].
As there are no clear recommendations and no consistent approach to the use of IV iron in patients with cancer in clinical practice, we undertook this systematic review and meta-analysis.
Material and methods
Data sources
We conducted a comprehensive search to identify trials in PubMed (January 1966 to August 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 3 of 4, July 2011), and the following conference proceedings for trials in oncology and haematology (2002–August 2011): Annual Meeting of the American Society of Hematology (ASH), Annual Meeting of the European Haematology Association (EHA), the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO). In addition we searched databases of ongoing and unpublished trials: http://www.controlled-trials.com, http://www.clinicaltrials.gov/ct and http://clinicaltrials.nci.nih.gov.
We used the following search term: (iron OR sodium ferric gluconate OR iron dextran OR iron [MeSH] OR Iron-Dextran Complex [MeSH] OR ferric citrate OR Ferric Compounds [MeSH] OR oral* iron OR intravenous iron OR iv iron OR iron-gluconate OR ferrlecit OR iron-gluconate OR venofer OR iron-sucrose OR ferrous sulphate) AND (cancer [MeSH] OR chemotherapy or malignancy or tumor) AND (Anemia or anemia [Mesh]). For PubMed, we added the Cochrane highly sensitive search term for identification of clinical trials [Citation16]. The references of all identified studies were inspected for more trials.
Study selection
We included all randomised controlled trials comparing IV iron with either no iron or oral iron for the treatment of anaemia in cancer patients. Trials were included whether patients received chemotherapy or not, and whether patients received ESA or not. Any IV iron preparation was included. All types of malignancy were included. Trials were included regardless of publication status, date of publication and language.
Two reviewers (AG, BR) screened all references identified through our search strategy and applied inclusion criteria. For possibly relevant articles or in the event of disagreement between the two reviewers, we obtained and independently inspected the full text article.
Data extraction and quality assessment
Two reviewers (AG, BR) independently extracted data from included trials. In the event of disagreement between the two reviewers, a third reviewer (LV) extracted the data and results were attained by consensus. Authors of trials were contacted for missing data when necessary. Both reviewers independently assessed risk of bias in included trials. We used the Cochrane Collaboration’s tool for assessing risk of bias. We individually assessed the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data reporting, selective outcome reporting. We separately assessed each domain and graded it as low risk for bias, unclear risk (lack of information or uncertainty over the potential for bias), or high risk for bias according to the criteria specified in the Cochrane Handbook version 5.1.0. [Citation16]
Definition of outcomes
The primary outcomes were: rate of patients achieving a haematopoietic response, defined as haemoglobin (Hb) level increase by more than 2 g/dl or an increase above 12 g/dl, and the rate of patients who required blood transfusion during the study period. For analysis of the primary outcome we included only trials of CIA.
Secondary outcomes were divided to efficacy outcomes and safety outcomes. Efficacy outcomes included: absolute ferritin level and transferrin saturation (TSAT) level at the end of the trial or change in these values from baseline if absolutes value were unavailable, as recommended [Citation16]. We aimed to assess time to haematopoietic response. We also assessed the number of patients with improvement in any of the validated quality of life (QOL) scales for cancer: functional assessment of cancer therapy (FACT) score, 100 mm linear analogue scale (LASA) score or Symptom Distress Scale (SDS). Safety outcomes included: all-cause mortality at the end of follow-up, any adverse event, adverse events which were considered serious or required intervention, cardiovascular events and thromboembolic events. For analysis of secondary outcomes we included all trials.
Data synthesis and analysis
Our main analysis was IV iron vs. standard care (no iron or oral iron), for patients with CIA. We conducted separate analyses for trials that administered ESA and trials that did not. For all analyses, in trials in which the standard care group included separate arms of no iron or oral iron (three arm trials), we preferentially compared IV iron with the “no iron” arm. We also conducted a separate analysis of IV iron vs. oral iron. Dichotomous data were analysed by calculating the relative risk (RR) for each trial with 95% confidence intervals (CI) (Review Manager [RevMan], version 4.2 for Windows, The Cochrane Collaboration, Oxford, UK). For continuous variables, we obtained mean and standard deviation. We calculated weighted mean difference (WMD) for continuous variables reported on the same scale. WMD represents the weighted combination of absolute differences between the mean values in the two groups in a clinical trial. This summary statistic has the same unit of measurement as the variable measured. Absolute end values rather than change from baseline values were analysed preferentially. Where unavailable, we combined end values and change from baseline values.
We assessed heterogeneity of trial results by calculating a χ2-test of heterogeneity and the I2 measure of inconsistency. We used a fixed-effect model with the Mantel-Haenszel method for pooling trial results throughout the review unless statistically significant heterogeneity was found (p <0.10 or I2>50%), in which case we chose a random-effects model and used the DerSimonian and Laird method [Citation17]. We explored potential sources of heterogeneity through sub-group analyses of the primary outcome according to different baseline parameters. In addition, we conducted meta-regression, assessing the effect of the following variables in each study on effect estimates for the primary outcome: baseline ferritin level, baseline TSAT level, baseline Hb level and total iron dose administered. Meta-regression was performed on the log risk ratio (Comprehensive Meta Analysis, version 2.2; BioStat, Englewood, NJ). The regression slope with its standard error and significance are reported.
Results
The search yielded 1118 potentially relevant trials, of which 57 were considered for further investigation. Of these, 46 studies were excluded for various reasons (). In addition, two abstracts from conference proceedings were included.
Figure 1. Trial flow according to QUOROM (quality of reporting meta-analysis) showing flow of trials included in the meta-analysis.
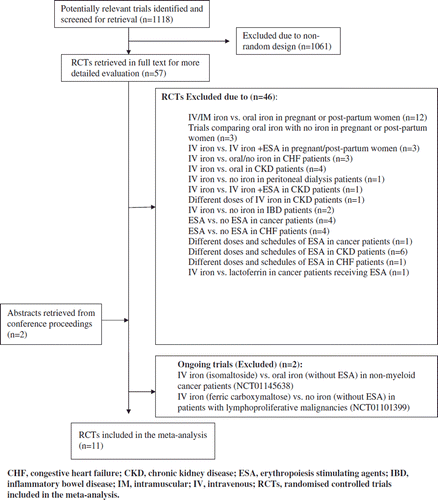
Eleven trials conducted between the years 2004 and 2011 and randomising 1681 patients fulfilled inclusion criteria. depicts the characteristics of included trials, and depicts the baseline iron status parameters.
Table I. Characteristics of included studies.
Nine trials used ESA and two trials did not, both of which were small trials conducted in gynaecologic cancer patients [Citation18,Citation19]. Chemotherapy was administered in all but one trial, which included indolent lymphoproliferative disorders [Citation11]. Most trials included mainly patients with solid tumours.
The baseline ferritin level in the trials ranged between 160 and 460 ng/ml (range 1–1000) (). The intervention was iron sucrose in five trials [Citation11,Citation18–21], ferric gluconate in three trials [Citation14,Citation22,Citation23], IV iron dextran in two trials [Citation24,Citation25], and one trial assessed both iron sucrose and ferric gluconate [Citation26]. The total IV iron dose in the trials ranged from 600 to 3000 mg, the average dose being approximately 1200 mg. The IV iron schedule varied between the trials. Iron was administered at a dose of either 100–125 mg every week, or 100–200 mg every two weeks, or 187.5–400 mg every three weeks (). IV iron was administered over a period of six to 16 weeks.
Table II. Baseline haematologic and iron status of included studies.
Risk of bias assessment showed that trials were of low risk for bias. Random allocation sequence was low risk of bias in seven of the trials and allocation concealment was low risk of bias in eight trials.
Primary outcome
Haematopoietic response. The main analysis of IV iron vs. standard care in patients with CIA receiving ESA demonstrated that IV iron significantly increased the rate of patients achieving a haematopoietic response [RR 1.28 (95% CI 1.125–1.45), seven trials, I2 = 68.1%, random effects model], (RR> 1 favours the IV iron arm, ).
Figure 2. Intravenous iron vs. standard of care: Rate of patients who achieved a haematopoietic response. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. CI, confidence interval; IV, intravenous; RR, relative risk; STD, standard.
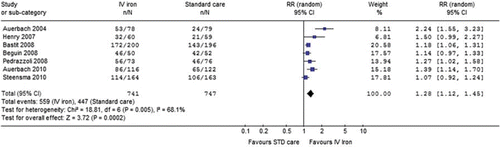
Sensitivity analysis including the single trial without chemotherapy showed similar results [RR 1.31 (95% CI 1.15–1.49), eight trials, I2 = 68.4%, random effects model]. There were no data regarding haematopietic response in trials without ESA.
We analysed haematopoietic response in different trial settings (): there was a significant increase in the rate of patients with a haematopoietic response in trials of chemotherapy and the one with no chemotherapy, and in trials of solid tumours.
Table III. Subgroup analyses of primary outcome, haematopoietic response (IV iron vs. standard of care).
When analysed according to type of IV iron preparation, the effect estimates for an increase in the rate of patients with a haematopoietic response for all iron preparations were similar, although significant only for iron sucrose.
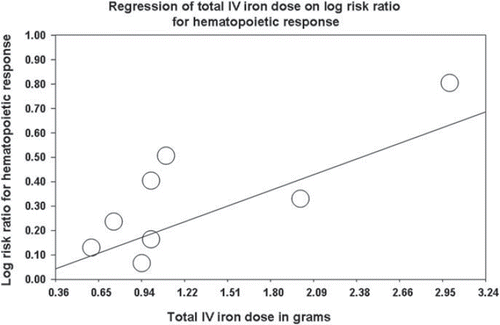
Meta-regression showed a statistical significant correlation between the total IV iron dose and the log risk ratio for haematopoietic response, with a change in the iron dose of 1 g resulting in a change of 1.25 (95% CI 1.10–1.42) in the risk ratio for haematopoietic response, p = 0.00056, ). However, we did not observe an effect of the baseline ferritin, TSAT or baseline Hb level on effect estimates by meta-regression.
We analysed separately the trials of CIA in which IV iron was compared to no iron [RR 1.21(95% CI 1.12–1.31), six trials, random effects model], and the trials in which IV iron was compared to oral iron [RR 1.37 (95% CI 0.92–2.05), three trials, random effects model].
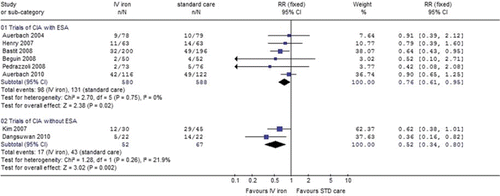
Transfusion requirements. The main analysis of IV iron vs. standard care in patients with chemotherapy induced anaemia receiving ESA demonstrated that IV iron significantly decreased the rate of patients who required blood transfusions [RR 0.76 (95% CI 0.61–0.95), seven trials]. Similar results were shown in the two trials without ESA [RR 0.52 (95% CI 0.34–0.80)], , (RR< 1 favours the IV iron arm).
Figure 4. Intravenous iron vs. standard of care: Rate of patients who required blood transfusions. Black squares represent the point estimate, their sizes represent their weight in the pooled analysis, and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. CI, confidence interval; IV, intravenous; RR, relative risk; STD, standard.
Secondary outcomes
Ferritin level at the end of the trial was significantly increased in the IV iron arm compared with the standard care [WMD 360.18 (95% CI 179.64–540.73), random effects model, six trials], as was TSAT [WMD 6.61 (95% CI 1.57, 11.65), random effects model, five trials].
Five trials reported time to haematopoietic response. Four trials reported time to response in medians [Citation11,Citation23,Citation25,Citation26] and one reported in means [Citation24]. The median time to response for the standard care group ranged between 46 and 94 days and the median time to response in the IV iron group ranged between 36 and 54 days.
Six trials reported QOL outcomes. For the pooled analysis of QOL we included trials which reported the number of patients with an improvement in FACT-Fatigue scale (a clinically significant increase was usually regarded as a ≥ 3 point increase [Citation22,Citation25,Citation26] and trials that reported improvement in the SDS-Fatigue scale [Citation14]. There was a significant increase in the number of patients with improvement in QOL scales for cancer in the IV iron arm [RR 1.25 (95% CI 1.05, 1.49), four trials, I2 = 71%, random effects model].
Safety
There was no difference in all-cause mortality at the end of follow-up between the IV iron arm and the standard care arm [RR 1.13 (95% CI 0.75, 1.70), seven trials, 1470 patients].
There was no difference in the rate of any adverse event [RR 0.99 (95% CI 0.93, 1.04), four trials], adverse events that required discontinuation of iron [RR 1.01 (95% CI 0.59, 1.70), four trials], or serious adverse events requiring intervention [RR 1.06 (95% CI 0.89, 1.27), seven trials]. In addition, there was no difference in the occurrence of thromboembolic events [RR 1.03 (95% CI 0.59, 1.80), four trials] or of cardiovascular events [RR 1.08 (95% CI 0.65, 1.78), six trials].
Discussion
Our systematic review compiles all trials assessing IV iron treatment for patients with anaemia and cancer. The vast majority of data comes from trials where IV iron was added to ESA therapy for chemotherapy-induced anaemia. We demonstrated that treatment with IV iron for CIA was associated with a statistically significant increase of 28% in the rate of haematopoietic response, and a statistically significant decrease of 26% in the number of patients who require blood transfusions. In addition, there was a statistically significant increase in iron metabolism parameters (ferritin, TSAT) and in QOL scores.
Our main finding that IV iron improves anaemia is of great importance. Anaemia at presentation was previously found to be a negative prognostic factor in various malignancies, both solid and haematological [Citation27]. The increase in haematopoietic response may be associated with better long-term overall survival. However, it could not be assessed due to the short follow-up period of 15 to 18 weeks.
The second finding of a 24% reduction in transfusion requirements is clinically important. Blood transfusions are associated with various risks as acute reaction, transfusion related acute lung injury, volume overload, and infections [Citation4,Citation13,Citation28]. Therefore, reduction in transfusion requirement may minimise these risks.
The increase in haematopoietic response with IV iron was consistent in almost all settings, and was independent of baseline iron status. Moreover, meta-regression revealed a direct correlation between IV iron dose and haematopoietic response, suggesting better response with a higher dose. This is also in accordance with the results of the Steensma et al. trial [Citation14,Citation15], which is the largest trial included in the meta-analysis and the only trial that showed negative results. The planned iron dose was quite low (937.5 mg) and the actual administered dose was even lower (650 mg) [Citation29].
Trials differed in inclusion criteria regarding baseline iron parameters. Most trials excluded patients with iron deficiency. Of the nine trials that reported baseline iron status, only two trials allowed inclusion of true iron deficient patients [Citation24,Citation26], but their actual number in these trials was low. Despite the differences in baseline iron status, meta-regression demonstrated no association between baseline ferritin and TSAT and haematopoietic response.
Of all the different iron preparations the improved haematopoietic response was statistically significant for iron sucrose only. However, the effect estimates were quite similar, suggesting a class effect for IV iron.
The reduction in blood transfusions was evident irrespective of the use of ESA. Although the two trials without ESA included only 119 patients, a significant reduction of 48% in blood transfusions was observed. Nowadays there is controversy regarding the use of ESA in cancer patients. ESA treatment in patients with CIA demonstrated clear benefits in haematopoietic response and reduction in transfusions [Citation5]. Recently, FDA alerted physicians to an association of shortened survival with ESA treatment. This was based on several randomised controlled trials, two of which administered ESA to patients with anaemia and cancer, not receiving chemotherapy [Citation30,Citation31]. The largest individual patient data meta-analysis [Citation32] demonstrated an increase in mortality with ESAs in all patients (including both CIA and cancer without treatment) and a study level meta-analysis demonstrated both increase in mortality and venous thromboembolic events [Citation33]. However, another pooled analysis of individual patient level data from randomised trials of darbepoetin alfa for treatment of patients with CIA showed no increase in mortality or disease progression, and an expected increase in the risk for venous thromboemboli [Citation34]. In this analysis the increase in adverse outcomes was seen in both treatment arms (ESA or not) only in patients who required transfusions. While European guidelines still consider ESA use with caution in patients receiving chemotherapy [Citation6,Citation9], current American guidelines do not recommend ESA use when chemotherapy with curative intent is administered [Citation7,Citation8] and consider it mainly for chemotherapy with a palliative intent. Due to this unresolved issue, the pure platform to assess IV iron is in trials of chemotherapy-induced anaemia in which ESA is not administered, as in the two trials in our meta-analysis [Citation18,Citation19]. Due to the ESA controversy, especially regarding administration without chemotherapy, we decided to exclude the single trial in which chemotherapy was not given from the primary analysis.
Of note, recently another meta-analysis assessing iron supplementation for cancer patients was published. As in our meta-analysis, an increase in haematopoietic response and a reduction in transfusion rate was shown with IV iron. However, IV iron was assessed only as an adjunct to ESA [Citation35].
Our study shows improvement in quality of life (QOL) scores. QOL is an important outcome in clinical trials, and especially in trials of cancer patients [Citation36]. For the individual patient, the improved feeling of well being may be more important than the mere increase in Hb level.
Iron supplementation has been shown to allow reduction of ESA dosage in the setting of chronic kidney disease [Citation37]. However, this outcome was not reported in most trials. The single trial which assessed this issue, indeed found a decrease in the total ESA dose in the IV iron arm [Citation11] suggesting a potential benefit of IV iron.
Our review demonstrates no increase in adverse events in the IV iron arm compared to standard care, and no difference in mortality. This is consistent with a recent meta-analysis of IV iron for patients with chronic renal failure [Citation37]. However, this should be interpreted with caution due to a small sample size and a short follow-up.
Of note, our review showed no difference in the rate of thromboembolic events between the IV iron arm and the standard care arm. This is in concert with a recent post hoc analysis of the Henry et al. trial [Citation38] that showed that patients treated with IV iron were less likely to develop an elevated platelet count and even had a decrease in thromboembolic events. The authors suggest that ESA-induced thromboemboli may be related to thrombocytosis due to iron-restricted erythropoiesis, and the IV iron possibly has a platelet lowering effect.
Limitations
Several limitations of our analysis merit consideration. The included studies were heterogeneous regarding the type of patients, different types of malignancies and different chemotherapy regimens, different iron preparations, schedule, and total dose of IV iron administered, different control groups (oral iron or no iron), and different types of ESA and schedule. Moreover, the trials applied different inclusion criteria regarding baseline haematologic and iron status parameters, and did not report results separately for absolute iron deficient patients, functional iron deficient patients, and iron-replete patients. Therefore, we were unable to conduct subgroup analyses according to baseline iron status.
Our primary outcome was transfusion requirements, but transfusion use was not standardised in the trials included in our systematic review.
Our main analysis was the comparison of IV iron with standard care. In most trials the comparator was no iron, but in three trials [Citation14,Citation22,Citation24] it was oral iron. The separate analysis for these three trials of IV iron vs. oral iron showed a similar effect estimate, although not reaching statistical significance. Due to the small number of trials, our meta-analysis does not enable to draw conclusions regarding the efficacy of oral iron.
Another limitation regarding methodology is that none of the trials were blinded, leaving them open to observer bias.
Finally, the definition of the primary outcome (haematopoietic response) was an increase of Hb to greater than 12 g/dl. However, this goal is less relevant given current recommendation of guidelines to initiate ESA only when Hb concentration has decreased to less than 10 g/dl in order to decrease transfusions [Citation7].
Implications for practice
Our systematic review and meta-analysis supports the use of supplemental IV iron for treatment of CIA in patients with solid tumours, and concomitantly treated with ESA. Although based on only two small trials, IV iron also reduced transfusions in cancer patients receiving chemotherapy without ESA. The results mainly apply to patients without iron deficiency, since these were the majority of patients in the trials. Although optimal dosage is not clear yet, it appears that higher doses led to a greater haematopoietic response, without an increase in adverse events. Thus, it may be reasonable to use the higher dose range reported in the trials, at the range of 1 to 1.5 g total, given over six to 16 weeks.
Implications for research
The main question is whether IV iron alone improves clinical endpoints and needs further study. Thus, it may be worthwhile to conduct more randomised controlled trials of IV iron vs. no iron without ESA. Currently two randomised controlled trials assessing IV iron without ESA are recruiting patients [Citation39,Citation40].
Open questions still exist regarding the optimal formulation, doses, schedule and duration of IV iron. These should be compared in future trials.
Acknowledgements
We thank Dr. David Henry, Dr. Michael Hedenus, Dr. Yves Beguin, and Dr. David Steensma who responded to our letters and supplied additional data regarding their trials.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, . The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293–306.
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–23.
- Bohlius J, Weingart O, Trelle S, Engert A. Cancer-related anemia and recombinant human erythropoietin – an updated overview. Nat Clin Pract Oncol 2006;3:152–64.
- Wauters I, Vansteenkiste J. Erythropoiesis-stimulating agents in cancer patients: Reflections on safety. Expert Rev Clin Pharmacol 2011;4:467–76.
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, . Recombinant human erythropoietin and overall survival in cancer patients: Results of a comprehensive meta-analysis. J Natl Cancer Inst 2005;97:489–98.
- Schrijvers D, De Samblanx H, Roila F. Erythropoiesis- stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol 2010;21(Suppl 5):v244–7.
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, . American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol 2010;28:4996–5010.
- NCCN. Clinical Practice Guidelines in Oncology: Cancer- and chemotherapy-induced anemia, version 2. 2011. Available at: http://www. nccn.org/professionals/physician_gls/PDF/anemia.pdf 2011 (accessed 2012 February 1)
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, . EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007;43:258–70.
- Goodnough LT. Erythropoietin and iron-restricted erythropoiesis. Exp Hematol 2007;35:167–72.
- Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, . Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: A randomized multicenter study. Leukemia 2007;21:627–32.
- Hedenus M, Birgegard G. The role of iron supplementation during epoietin treatment for cancer-related anemia. Med Oncol 2009;26:105–15.
- Henry DH. Parenteral iron therapy in cancer-associated anemia. Hematology Am Soc Hematol Educ Program 2010; 2010:351–6.
- Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, . Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol 2010; 29:97–105.
- Steensma DP, Sasu BJ, Sloan JA, Tomita D, Loprinzi CL, . The relationship between serum hepcidin levels and clinical outcomes in patients with chemotherapy-associated anemia treated in a controlled trial. J Clin Oncol 2011 ASCO Annual meeting proceedings 2011;29(15 Suppl):9031.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions: version 5.1.0. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org (accessed 2011 December 1)
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
- Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol Oncol 2010;116:522–5.
- Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH, . Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol Oncol 2007;105:199–204.
- Beguin Y, Maertens J, De Prijck B, Schots R, Frere P, Bonnet C, . Darbepoetin-alfa and i. v. iron administration after autologous hematopoietic stem cell transplantation: A prospective randomized multicenter trial. Blood (ASH Annual Meeting Abstracts) 2008;112. Abstract 54.
- Bellet RE, Ghazal H, Flam M, Drelichman A, Gabrail N, Woytowitz D, . A phase III randomized controlled study comparing iron sucrose intravenously (IV) to no iron treatment of anemia in cancer patients undergoing chemotherapy and erythropoietin stimulating agent (ESA) therapy. J Clin Oncol 2007 ASCO Annual Meeting Proceedings 2007;25: 9109.
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12:231–42.
- Pedrazzoli P, Farris A, Del Prete S, Del Gaizo F, Ferrari D, Bianchessi C, . Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol 2008;26:1619–25.
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, . Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J Clin Oncol 2004;22:1301–7.
- Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu TE, Shao J, . Darbepoetin alfa 300 or 500 mcg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol 2010;85:655–63.
- Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, . Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 2008;26:1611–8.
- Clarke H, Pallister CJ. The impact of anaemia on outcome in cancer. Clin Lab Haematol 2005;27:1–13.
- Goodnough LT. Risks of blood transfusion. Anesthesiol Clin Nth Am 2005;23:241–52.
- Aapro M, Beguin Y, Birgegard G, Gascon P, Hedenus M, Osterborg A. Too-low iron doses and too many dropouts in negative iron trial?J Clin Oncol 2011;29:e525–6; author reply e527–8.
- Smith RE, Jr.,Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, . Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: Results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 2008;26:1040–50.
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, . Randomized, double-blind, placebo- controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007;25: 1027–32.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 2009;373: 1532–42.
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, . Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914–24.
- Ludwig H, Crawford J, Osterborg A, Vansteenkiste J, Henry DH, Fleishman A, . Pooled analysis of individual patient-level data from all randomized, double-blind, placebo- controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol 2009;27:2838–47.
- Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Addition of iron to erythropoiesis-stimulating agents in cancer patients: A meta-analysis of randomized trials. J Cancer Res Clin Oncol 2012;138:179–87.
- Husain A, Myers J, Selby D, Thomson B, Chow E. Subgroups of advanced cancer patients clustered by their symptom profiles: Quality-of-life outcomes. J Palliat Med 2011;14:1246–53.
- Rozen-Zvi B, Gafter-Gvili A, Paul M, Leibovici L, Shpilberg O, Gafter U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: Systematic review and meta-analysis. Am J Kidney Dis 2008;52:897–906.
- Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol 2012;87:308–10.
- The Clinical Trials. gov website, a service of the U.S National institutes of Health. Available from: http://clinicaltrials.gov/ct2/show/NCT01101399 (accessed 2012 January 17)
- The Clinical Trials.gov website, a service of the U.S National institutes of Health. Available from: http://clinicaltrials.gov/ct2/show/NCT01145638 (accessed 2012 January 17)