Abstract
Background. Spinal cord compression is an oncological and surgical emergency. Delays in referral and diagnosis may influence functional outcome. It is therefore important to identify patients who will regain or maintain the ability to walk after surgery. The aim of the present study was to examine current practice for referral and diagnosis of prostate cancer patients with spinal cord compression and to identify prognostic factors for neurological outcome after surgery. Patients and methods. The study includes 68 consecutive patients with prostate cancer who underwent surgery due to neurological compromise. Intervals from onset of neurological symptoms to referral, diagnosis, and treatment were analyzed in relation to functional outcome. The prognostic significance of preoperative clinical parameters on gait function one month after surgery was evaluated. Results. Patients who were referred from local hospitals had longer delay to surgery than those who directly presented to the cancer center (p = 0.004). The rate of diagnosis with MRI increased through the week and peaked on Friday, with few patients being diagnosed during weekends. The ability to walk before surgery, hormone-naive prostate cancer, and/or shorter time from loss of ambulation were associated with more favorable neurological outcome. In patients with hormone-refractory disease who were unable to walk before surgery regaining ambulation was associated with: duration of paresis < 48 hours (p = 0.005), good preoperative performance status (p = 0.04), preoperative PSA serum level < 200 ng/ml (p = 0.03), and surgery with posterior decompression and stabilization (p = 0.03). Conclusion. Early diagnosis and rapid treatment of spinal cord compression in prostate cancer patients is crucial for neurological recovery. Raising awareness of the condition among patients at risk and among physicians is of outmost importance as well as improving local and regional guidelines for treatment.
Spinal cord compression is one of the most devastating complications of metastatic prostate cancer, causing profound disability due to pain and loss of neurological function. Men with prostate cancer have approximately a 7% risk of developing clinically symptomatic spinal cord compression [Citation1]. One third of patients with vertebral metastases have clinically occult spinal cord compression on MRI [Citation2,Citation3]. Improvement in diagnosis and treatment of spinal cord compression would have an important impact on morbidity and quality of life in many patients.
Spinal cord compression is an oncological and surgical emergency [Citation4]. The neurological function before treatment is the most important prognostic factor for functional outcome [Citation5,Citation6]. It is important to make a diagnosis and start the treatment while the patients are still walking. Several studies have demonstrated substantial delays in referral, diagnosis, and treatment of patients with spinal cord compression [Citation7–9]. In these studies, a high proportion of patients have lost their ambulation capacity at the time of treatment. Thus, awareness of early symptoms of spinal cord compression and modern guidelines for treatment make a difference for the patient [Citation10,Citation11].
The aims of this study were to examine current practice for referral and diagnosis of prostate cancer patients with spinal cord compression and to identify clinical parameters that influence neurological recovery after surgery.
Methods
We retrospectively reviewed the records of 68 consecutive patients with prostate cancer who underwent surgery for metastatic spinal cord compression at Umeå University Hospital, Sweden, between September 2003 and September 2010 (). The study was approved by the local ethics review board of Umeå University (No. 223/03, dnr 03-185 and dnr 04-26M).
Table I. Clinical characteristics of 68 patients with prostate cancer who underwent surgery for metastatic spinal cord compression (MSCC).a
The indication for surgery was neurological deficit. Details regarding the treatment of prostate cancer before and after surgery for spinal cord compression, complications to surgery, survival, and morphology of bone metastases have been described previously [Citation12–14]. Functional outcome was assessed one month after surgery. Patients who died within one month after surgery were included with the functional outcome achieved before death. Grading of neurological function according to the Frankel scale and evaluation of Karnofsky Performance Status scale (KPS) in this patient material have been described in our previous studies [Citation13,Citation14].
The data on time for admission to hospital, MRI scan, and surgery were extracted from hospitals records. Time intervals to diagnosis and treatment were expressed in terms of whole days according to Husband [Citation7], where a delay of < 24 hours = 0 days, ≥ 24 < 48 hours = 1 day, etc.
Statistical analysis
Two independent samples were compared with the Mann-Whitney U-test and proportions with the Fisher's exact test. A p-value of ≤ 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Inc., San Diego, CA, USA) software.
Results
Patients initially presented with signs of spinal cord compression to the local hospital in 55 (81%) cases and directly to the cancer center in 13 (19%) cases. The delays from the first contact with the hospital to surgery and the delays from MRI diagnosis to surgery were shorter if the patient first presented directly to the cancer center rather than to the local hospital ().
Table II. Delay to surgery for spinal cord compression.a
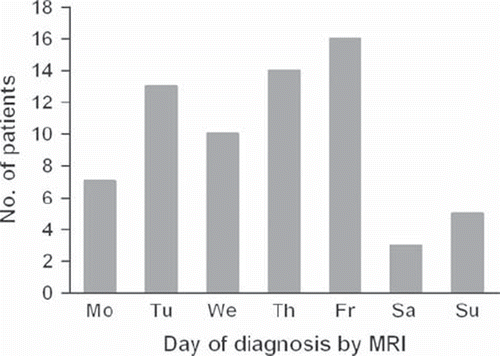
Interestingly, the number of MRI investigations increased through the week being maximal on a Friday (). In 79% (54/68) of patients MRI was performed within one day either before or after admission to the cancer center (). In 71% (48/68) of patients surgery was performed the same or next day as the MRI (). Median interval between admission to the cancer center and surgery was 19 hours (). In 35 of patients surgery started during normal working time (08.00–16.00) whereas in 33 patients surgery started out of hours (16.00–08.00).
Table III. Time interval between MRI and cancer center admission.
Table IV. Time interval between MRI and surgery.
There was no statistically significant difference in intervals to diagnosis and surgery between the patients with previous history of prostate cancer (hormone-refractory) and those in whom the spinal cord compression was the first sign of previously unknown cancer (hormone-naïve) ().
Generally, ability to walk before surgery was associated with better neurological outcome, whereas hormone-naïve prostate cancer and/or shorter time from loss of ambulation were related to better functional recovery among patients who were non-ambulatory before surgery ().
Table V. Clinical features influencing functional status after surgery.
Patients with hormone-refractory cancer which were non-ambulatory before surgery had less favorable functional outcome. In these patients, factors associated with regaining of ambulation were: duration of paresis < 48 hours, good preoperative performance status (KPS 80–100%), preoperative PSA serum levels < 200 ng/ml, and surgery with posterior decompression and stabilization ().
Table VI. Prognostic parameters for postoperative functional status in hormone-refractory patients that were non-ambulatory before surgery (n = 46).
The functional outcome in studies reporting treatment of metastatic spinal cord compression in prostate cancer is shown in .
Table VII. Series reporting treatment of metastatic spinal cord compression in prostate cancer.
Discussion
Prostate cancer patients with bone metastasis are at risk of developing spinal cord compression. It is important that both patients and physicians are aware of this condition. Our results suggest that surgery was performed too late in some of the patients. The delays in diagnosis and treatment may have influenced the neurological outcome.
Delays in diagnosis and treatment
Although the importance of early diagnosis and treatment of metastatic spinal cord compression has been known for a long time there is still evidence that this condition may be easily missed at first presentation [Citation15], leading to a high proportion of patients that cannot walk at the time of diagnosis [Citation16]. As the ability to walk before treatment is the most important predictor of functional outcome [Citation5,Citation6], making diagnosis and starting the treatment before development of irreversible neurological deficits is of paramount importance for the quality of life of the patients [Citation17].
We found that patients who were referred from local hospitals had longer delays to diagnosis and treatment than the patients who initially presented directly to the cancer center. Partially, these delays could be related to the geographic specificity of Northern Sweden, which includes a huge area and long winters. However, the delays in referral may also be the consequence of unclear referral pathways from local hospitals to the cancer center. Furthermore, our data may suggest that the delays in diagnosis were related to the scarcity of information given to patients. It would be reasonable to expect shorter delays to diagnosis and treatment of spinal cord compression in patients with a previous history of cancer [Citation7,Citation8], but this was not the case in the present study. Indeed, almost half of our patients with hormone refractory tumors had been hesitating more than one week before contacting their hospital in spite of neurological symptoms. Several authors have pointed out the need for improving awareness regarding early symptoms of spinal cord compression both among patients and in primary and secondary care providers [Citation7,Citation8,Citation10]. This is particularly important for patients with a previous history of prostate cancer who have a high incidence of occult spinal cord compression in the absence of neurological symptoms [Citation2,Citation3].
Although the majority of patients in our study were referred from local hospitals only less than half of them had undergone an MRI scan prior to admission to the cancer center. Similar results were reported in a retrospective audit of clinical practice at a UK regional cancer center [Citation9]. Like Poortmans et al. [Citation18] and Levack et al. [Citation8], we found that the rate of diagnosis with MRI peaked on Friday, with few patients being diagnosed during weekends. Thus, besides delays in referral and diagnosis, our results may also indicate low availability of MRI scans for this group of patients. Hence, one of the main findings in this study is that there is still need both for optimizing of referral and improvement of access to MRI for patients with spinal cord compression. This needs to be addressed particularly in light of a study by Allan et al. [Citation11] that has showed that fast-track referral system with rapid access to MRI reduces the interval to diagnosis, leading to better ambulatory status at diagnosis.
The present study also highlights the problem of limited resources at surgery. Surprisingly half of the operations started out of hours. Still, the median interval from admission to the cancer center to surgery was 19 hours. Although spinal cord compression represents a surgical emergency, it appears that in our hospital other surgical emergencies have higher priority. Jansson and Bauer [Citation19] reported on a similar patient group but 90% of their patients underwent surgery during the daytime, preferably within 24 hours from admission.
Prognostic factors
Our patients with hormone-naïve prostate cancer had better neurological outcome than the patients with hormone-refractory tumors. This may be explained by the sensitivity of spinal metastases to androgen ablation [Citation12], but also by the better general condition of these patients leading to better total outcome after surgery [Citation13,Citation14]. Our results are in line with other studies showing that the functional outcome for these patients was better if they were treated by surgery followed by radiotherapy [Citation20,Citation21] than after radiotherapy alone [Citation22] or hormone therapy alone [Citation21].
In studies reporting treatment of spinal cord compression in prostate cancer 75–100% of ambulatory patients preserved their functional status irrespective of treatment modality [Citation20,Citation22–25] (). This was also the case in the present study where all preoperatively ambulant patients retained their ability to walk after surgery. Furthermore, half of our non-ambulatory patients regained ambulatory status at one month after surgery. Other studies showed that non-ambulatory patients regained their ability to walk in 67% cases after surgical treatment [Citation23], whereas this proportion was 57–63% in mixed series [Citation20,Citation24] and 33% in a study of radiotherapy alone [Citation22].
In this study we analyzed neurological outcome at one month after surgery. The routine in our institution was to give adjuvant radiotherapy approximately one month after surgery [Citation13]. Therefore, we believe that the functional status at one month reflects primarily the effect of surgery. However, the effect of adjuvant radiotherapy may account for high proportion of our patients who preserved ambulatory status at six months [Citation13]. Recently, a prospective, randomized study has shown that surgery followed by radiotherapy is superior for both preservation and regaining of walking ability compared to radiation alone [Citation26]. This study caused major breakthrough in favor of surgery and is frequently cited as a guide of current management of patients with metastatic spinal cord compression. However, the median survival was only 4.2 months, in spite of meticulous selection criteria [Citation26]. This fact further emphasizes the importance of careful selection of patients who may benefit from surgery.
In our hormone-refractory patients, duration of paresis of less than 48 hours was associated with regaining ambulatory status after surgery. The impact of duration of neurological symptoms on functional outcome is still controversial. Rades et al. analyzed the results of radiotherapy for spinal cord compression in patients with various tumors [Citation27] and with prostate cancer [Citation22]. They found significantly better functional outcome in patients whose motor deficits developed slowly (more than 14 days better than 8 to 14 days and 1 to 7 days). Slow progress of motor deficits may indicate more reversible spinal cord injury with more ambulatory patients at the start of treatment [Citation27]. On the contrary, prolonged paresis may indicate more irreversible injury, which may be the case in some patients with hormone-refractory prostate cancer in the present study. Only a few retrospective studies have reported that shorter duration of paresis is related to better neurological outcome after surgical treatment of spinal cord compression. Patients who presented with paresis for less than 48 hours [Citation28,Citation29] or less than 72 hours [Citation30] had increased likelihood of recovering ambulation. The retrospective character of these studies, the low number of patients, and various tumors included, make it difficult to draw conclusions on specific tumor types.
Hormone-refractory patients who underwent posterior decompression and stabilization had better functional outcome than those with posterior decompression only. Although this is a retrospective study with non-randomized treatment it seems reasonable that the patients with initially better general condition were chosen for stabilization, which may have influenced the results. It is also possible that the known osteoblastic nature of skeletal prostate cancer metastasis misled surgeons to consider the spine as stable and thus opt for only posterior decompression in patients in worse general condition.
A limitation of our study is the fact that data on pain assessment were missing or were not suitable for analysis. Although this does not imply that pain was not assessed and managed adequately it may be a further indication of low awareness among physicians for symptoms of spinal cord compression. Due to the retrospective character of the study medical and surgical treatments were not randomized but were determined according to the preference of clinicians, based on their individual experience as well as on institutional resources.
In conclusion, the hormone status of prostate cancer and pretreatment ability to walk influence neurological outcome after surgery for spinal cord compression. Moreover, our results suggest that delays in diagnosis and treatment have negative impact on functional outcome. This implicates not only the need for improvement of local and regional guidelines for treatment of patients with metastatic spinal cord compression, but also the importance of informing the patients at risk.
Acknowledgements
We would like to thank Kari Ormstad for linguistic revision. Inger Lindström, Pernilla Andersson, Elisabeth Dahlberg, and Birgitta Ekblom provided skillful technical assistance. This work was supported by grants from the Swedish Cancer Society and the County Council of Vasterbotten.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Loblaw A, Mitera G. Malignant extradural spinal cord compression in men with prostate cancer. Curr Opin Support Palliat Care 2011;5:206–10.
- Bayley A, Milosevic M, Blend R, Logue J, Gospodarowicz M, Boxen I, et al. A prospective study of factors predicting clinically occult spinal cord compression in patients with metastatic prostate carcinoma. Cancer 2001;92:303–10.
- Venkitaraman R, Sohaib SA, Barbachano Y, Parker CC, Khoo V, Huddart RA, et al. Detection of occult spinal cord compression with magnetic resonance imaging of the spine. Clin Oncol 2007;19:528–31.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol 2005;23:2028–37.
- Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: A prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys 2000;46:1163–9.
- Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008;7:459–66.
- Husband DJ. Malignant spinal cord compression: Prospective study of delays in referral and treatment. BMJ 1998; 317:18–21.
- Levack P, Graham J, Collie D, Grant R, Kidd J, Kunkler I, et al. Don't wait for a sensory level-listen to the symptoms: A prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol 2002;14:472–80.
- McLinton A, Hutchison C. Malignant spinal cord compression: A retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer 2006;94:486–91.
- White BD, Stirling AJ, Paterson E, Asquith-Coe K, Melder A. Diagnosis and management of patients at risk of or with metastatic spinal cord compression: Summary of NICE guidance. BMJ 2008;337:a2538.
- Allan L, Baker L, Dewar J, Eljamel S, Grant RM, Houston JG, et al. Suspected malignant cord compression – improving time to diagnosis via a ‘hotline’: A prospective audit. Br J Cancer 2009;100:1867–72.
- Crnalic S, Hörnberg E, Wikström P, Lerner UH, Tieva A, Svensson O, et al. Nuclear androgen receptor staining in bone metastases is related to a poor outcome in prostate cancer patients. Endocr Relat Cancer 2010;17:885–95.
- Crnalic S, Hildingsson C, Wikström P, Bergh A, Löfvenberg R, Widmark A. Outcome after surgery for metastatic spinal cord compression in 54 patients with prostate cancer. Acta Orthop 2012;83:80–6.
- Crnalic S, Löfvenberg R, Bergh A, Widmark A, Hildingsson C. Predicting survival for surgery of metastatic spinal cord compression in prostate cancer: A new score. Spine 2012 May 29 [Epub ahead of print].
- Quraishi NA, Esler C. Metastatic spinal cord compression. BMJ 2011;342:d2402.
- Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg [Br] 2010;92-B:1054–60.
- Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “All I care is walking and living my life”. JAMA 2008;299:937–46.
- Poortmans P, Vulto A, Raaijmakers E. Always on a Friday? Time pattern of referral for spinal cord compression. Acta Oncol 2001;40:88–91.
- Jansson K-Å, Bauer HCF. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J 2006;15:196–202.
- Huddart RA, Rajan B, Law M, Meyer L, Dearnaley DP. Spinal cord compression in prostate cancer: Treatment outcome and prognostic factors. Radiother Oncol 1997;44: 229–36.
- Nagata M, Ueda T, Komiya A, Suzuki H, Akakura K, Ishihara M, et al. Treatment and prognosis of patients with paraplegia or quadriplegia because of metastatic spinal cord compression in prostate cancer. Prostate Cancer Prostatic Dis 2003;6:169–73.
- Rades D, Staplers LJA, Veninga T, Rudat V, Schulte R, Hoskin PJ. Evaluation of functional outcome and local control after radiotherapy for metastatic spinal cord compression in patients with prostate cancer. J Urol 2006;175:552–6.
- Williams BJ, Fox BD, Sciubba DM, Suki D, Tu SM, Kuban D, et al. Surgical management of prostate cancer metastatic to the spine. J Neurosurg Spine 2009;10:414–22.
- Tazi H, Manunta A, Rodriguez A, Patard JJ, Lobel B, Guille F. Spinal cord compression in metastatic prostate cancer. Eur Urol 2003;44:527–32.
- Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop 2012;83:74–9.
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomized trial. Lancet 2005;366:643–8.
- Rades D, Heidenreich F, Karstens JH. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2002; 53:975–9.
- Fürstenberg CH, Wiedenhöfer B, Gerner HJ, Putz C. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg (Br) 2009;91-B:240–4.
- Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 2008;62:683–92.
- Hessler C, Burkhardt T, Raimund F, Regelsberger J, Vettorazzi E, Madert J, et al. Dynamics of neurological deficit after surgical decompression of symptomatic vertebral metastases. Spine 2009;34:566–71.