Abstract
Background. Cancer among adolescents and young adults (AYAs; 15–29 years old) is relatively rare but its incidence is increasing worldwide. To define the extent and nature of the AYA patients, this population-based study was performed to explore trends in cancer incidence, survival and risk of second primary cancers in AYAs. Material and methods. Data from all AYAs diagnosed with cancer between 1989 and 2009 were obtained from the Netherlands Cancer Registry. Age-standardized incidence rates with estimated annual percentage of change (EAPC) and five-year relative survival rates were calculated. Relative survival was used as a good approximation of cause-specific survival. All analyses were stratified by gender, five-year age group and calendar period. In addition, Standardized Incidence Ratios were determined to evaluate the risk of second primary cancers. Results. 23 161 AYAs were diagnosed with cancer between 1989 and 2009. Since 1989 the cancer incidence has increased significantly from 28 to 43 per 100 000 person years in males (EAPC: 1.9) and from 30 to 40 per 100 000 person years in females (EAPC: 1.4). The most frequently diagnosed cancers in male AYAs included testicular cancer, melanoma and Hodgkin's disease, whereas in females melanoma, breast cancer and Hodgkin's disease were the most frequently occurring cancers. Five-year relative survival rates were 80% and 82% for males and females, respectively. Over time, the five-year relative survival increased from 74% to 86% and from 79% to 86% in males and females, respectively. The risk of developing a second primary cancer was increased three to six times in males and two to five times in females, depending on rules for counting second primary cancers. Conclusions. Although the overall survival has improved over time, the progress made in AYAs for specific cancers is still less compared to improvements made in children and adults. This and the increasing incidence and high risk of second primary cancers warrants further research.
Background
Cancer in adolescents and young adults (AYAs) with an age at diagnosis between 15 and 29 years is relatively rare but its incidence is increasing [Citation1–4]. The age-adjusted incidence rate of cancer (European Standardized Rate; ESR) in AYAs in the Netherlands was 41 per 100 000 person years in 2009. Compared to the total incidence of cancer in the Netherlands, AYAs only represent a small part, i.e. slightly over 1%. However, compared to cancer incidence in children (ESR, < 15 years) which was 12 per 100 000 person years in 2009, the incidence among AYAs is more than three times higher (http://www.cancerregistry.nl). Former studies showed that the progress made in survival of AYAs with cancer is limited as compared to the steadily increase in survival in adults and the large improvements that have been achieved in many childhood cancers [Citation5,Citation6]. This lack of survival improvement might be due to differences in biological characteristics or etiology. Insufficient tailor-made professional attention for AYAs with cancer and lack of specialized guidelines may also be responsible for suboptimal outcome in this particular subset of young cancer patients.
Furthermore, although the improvement in survival of AYAs with cancer lags behind, the majority of the patients survive their cancer and attention should be paid to their lifetime risk of developing second primary cancers. In a small Dutch study including patients diagnosed between 1989 and 2003 at the age of 12–24 years in the Northern part of the Netherlands, a strongly increased risk of developing a second primary cancer was found [Standardized Incidence Ratio SIR: 31, 95% Confidence Interval (CI): 20.0–44.8] [Citation7].
In order to improve the current care of AYAs with cancer and to develop services tailored to their needs, it is important to define the extent and nature of this patient population by use of analyses of population-based, accurate and recent data. As recent, population-based information on the cancer occurrence in AYAs is scarce, we performed the current study including all cancer patients diagnosed at the age of 15 to 29 years between 1989 and 2009 in the Netherlands in order to give an extensive overview. Cancer-specific trends in incidence, survival and risk of second primary cancers in the AYA population were evaluated by gender, age group and calendar period.
Material and methods
Data were obtained from the Netherlands Cancer Registry (NCR), a nationwide population-based registry that includes all new diagnoses of cancer since 1989 except for basal cell carcinomas of the skin. The completeness of the Netherlands Cancer Registry since 1989 is estimated to be over 95%. All patients diagnosed with a primary malignant cancer at the age of 15 to 29 years in the period 1989 to 2009 were included. Benign tumors, tumors of uncertain malignancy (including borderline ovarian tumors, myelodysplastic syndromes, carcinoid of the appendix) were excluded. Patient- and tumor characteristics such as age at diagnosis, date of diagnosis, topography, morphology, invasiveness, WHO grade of differentiation, lateralization and stage as well as follow-up information concerning vital status and second primary cancers were retrieved. Vital status of all patients recorded in the NCR is updated annually by record linkage to the Dutch Municipal Personal Records Database which keeps information about vital status of all inhabitants in the Netherlands. All cancers in the NCR are classified using the International Classification of Diseases for Oncology (ICD-O). Cancers diagnosed between 1989 and 1992 were coded using the first edition of the ICD-O. Between 1993 and 2000 the second edition of the ICD-O was used and since 2001 the ICD-O third edition. Although, this classification on topography can be used for all cancers, it is largely satisfactory for late age of onset cancers, which mainly exist of carcinomas. As in young people the carcinomas only represent a small part of all diagnosed cancers a classification based on the histology is more appropriate to use. In the current study all primary tumors were therefore re-classified using the histology-based classification scheme as proposed by Birch et al. [Citation8] which gives an accurate and balanced overview of cancers in the younger age groups.
Cancer incidence
Cancer incidence rates were calculated by gender, five-year age groups, and calendar period (1989–1995, 1996–2002 and 2003–2009). Rates were age-adjusted by standardization to the European standard population (European Standardized Rates, ESR). Gender, age and calendar year specific population data were annually retrieved from Statistics Netherlands (http://statline.cbs.nl).
Relative survival
As the cause of death of the patients is not available in the cancer registry, disease specific survival could not be calculated. Instead, relative survival analyses were performed according to Dickman as a good approximation of disease-specific survival [Citation9]. This method adjusts crude survival rates amongst cancer patients for the expected mortality according to annual life tables of the general population matched on age, gender and calendar period (as annually retrieved from Statistics Netherlands). In the survival analyses end of follow-up was defined as date of death, date of emigration or 1 January 2010 whichever came first. Five-year tumor specific relative survival rates by gender and age group were calculated. To evaluate trends over time in survival, five-year survival rates were calculated for the top 10 most frequently diagnosed tumors in males and females by calendar period (1989–1993, 1994–1998, 1999–2003, 2004–2009) as well.
Risk of second primary cancers
All analyses on second primary cancers only included primary cancers, i.e. recurrences or progressive disease were not included in the analyses. In order to evaluate the risk of second primary cancers, SIRs with 95% CIs assuming a Poisson distribution were calculated. Person-years at risk were accumulated for each person in the cohort from the date of diagnosis of the first primary cancer up to the date of diagnosis of second primary cancer, date of death, date of emigration or 1 January 2010, whichever came first. The expected number of second primary cancers was calculated by applying the five-year age, calendar year and gender-specific incidence rates for each cancer in the Netherlands to the person-years at risk among the AYA cohort. SIRs were calculated as the ratio of the observed number of second primary cancers and the expected incident number of given tumors in a specified gender- and age group.
All analyses on second primary cancers were performed three times. Once including all second primary cancers, regardless of the time between first and second primary cancer and type of cancer. In this way we were able to evaluate the risk of developing a second primary cancer, including the contra-lateral or same site tumors (for example for testicular cancer, breast cancer and melanoma). Secondly, in order to exclude the effect of detection bias, cancers presented concurrently at the time of first diagnosis (rather than subsequent to the first diagnosis) and second primary cancers diagnosed within three months of the first cancer were excluded. Thirdly, in order to be able to compare the calculated SIRs with results reported by other studies, the International rules concerning multiple cancers as proposed by the International Agency for Research on Cancer/International Association of Cancer Registries (IARC/IACR) were applied [Citation10]. Using this latter definition the recognition of a second primary cancer is independent of time and only one primary tumor arising in an organ as defined by the three character topography code can be considered as primary cancer. Analyses were performed using the statistical software package SAS 9.2.
Results
Overall 23 161 AYAs were diagnosed with cancer between 1989 and 2009 in the Netherlands. Fifty-one percent were male, 18% were diagnosed between 15 and 19 years, 30% between 20 and 24 years and 52% between 25 and 29 years.
Cancer incidence over time
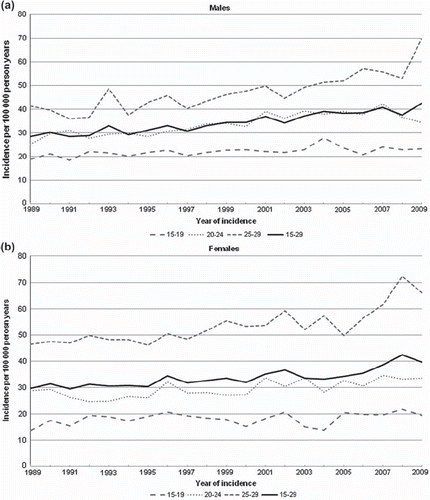
displays the cancer incidence rates by tumor type, gender and by period () and age (). Incidence rates were based on tumors instead of patients (n = 23 360 tumors diagnosed between 1989 and 2009, at age 15–29 years). Overall, the cancer incidence among male AYAs has increased sharply since 1989. The most prominent trend over time concerned testicular cancer (gonadal germ cell tumor) in males; testicular cancer incidence has doubled from 7 per 100 000 person years in 1989–1995 to 14 per 100 000 in 2003–2009 (EAPC = 4.90, p < 0.01). When excluding testicular cancer a moderate rise in overall cancer incidence is still evident. Significant rising trends (p < 0.01) were seen for chronic myeloid leukemia (CML), Hodgkin's disease, head and neck carcinomas and carcinomas of the respiratory tract. A decreasing trend was observed for astrocytoma. In females, a clear increase in cancer incidence is seen as well; significant increases were observed for CML, Hodgkin's disease, melanoma, thyroid cancer and breast cancer. Astrocytoma and gonadal germ cell tumors were significantly decreased. In the trends in cancer incidence over time by age and gender are presented. Over time the incidence of cancer among AYAs has increased significantly (EAPC = 1.88, p < 0.01 for males and EAPC = 1.37, p < 0.01 for females).
Table I. Age-standardized incidence rates of cancer in AYAs by tumor and gender.
Cancer incidence by age
Within the AYA population, the incidence increased sharply with age. Each five-year age category showed a different pattern of observed cancer types. In male AYAs with age 15 to 19 years testicular cancer was the most frequently observed cancer (19%), followed by Hodgkin's disease (15%) and non-Hodgkin lymphoma (9%). In 20–24 year olds testicular cancer comprised one-third of all cancers, followed by Hodgkin's disease (13%) and melanoma (12%). In the oldest group (age 25–29 years), testicular cancer was still diagnosed in 35% of all patients. Melanoma (14%) and Hodgkin's disease (9%) were second and third most frequent cancers.
In female AYAs with age 15 to 19 years Hodgkin's disease (21%), melanoma (17%) and non-Hodgkin's lymphoma (7%) were most frequently diagnosed. In females aged 20 to 24 years melanoma accounted for 29% of all cancers, followed by Hodgkin's disease (14%) and thyroid cancer (6%). In the oldest female AYAs (25 to 29 years) melanoma still represented the most frequent tumor (28%) with breast cancer (17%) and cancer of the genito-urinary tract (mainly cervical cancer) (13%) as number two and three.
Relative survival
In , the five-year tumor specific relative survival rates by gender and age are presented. Overall, the five-year relative survival in males was slightly worse compared to females (80% vs. 82%, p < 0.001). A fairly good survival (at least 80%) was observed in male patients with Hodgkin's disease, ependymoma, chondrosarcoma, other bone tumors, fibrosarcoma, gonadal germ cell tumors, melanoma, non-melanoma skin cancer, thyroid cancer, head and neck cancer and cancer of the genito-urinary tract ( > 80% was kidney or bladder cancer). Patients with acute myeloid leukemia (AML), acute lymphatic leukemia (ALL), other leukemia, other central nervous system tumors, Ewing's tumor, osteosarcoma, rhabdomyosarcoma, and unspecified malignant neoplasms fare worse with a five-year survival of approximately 50% or less. Largely similar to males, a five-year survival of at least 80% was seen in female patients with Hodgkin's disease, chondrosarcoma, other bone tumors, fibrosarcoma, gonadal germ cell tumors, non-gonadal germ cell tumors, melanoma, non-melanoma skin cancer, thyroid cancer, head and neck cancer and cancer of the genito-urinary tract. Female patients with AML, central nervous system tumors, Ewing's tumor, rhabdomyosarcoma, and unspecified malignant neoplasms have a fairly poor survival of less than 50%. In addition, the five-year relative survival over time was evaluated (data not shown). Since 1989, a distinct improvement in the overall relative survival of approximately 74% to 86% in males and 79% to 86% in females was observed. A clear survival improvement in patients with non-Hodgkin lymphoma in both males and females was seen. Among males, the survival of gonadal germ cell tumors and melanoma showed a slight but steady increase over time. The five-year survival rates of the majority of other cancers (with the exception of Hodgkin's disease and AML) seemed to be improved as well although the 95% CIs are wide. In contrast to males, females showed a minor survival improvement for Hodgkin's disease. The survival of female breast cancer patients improved significantly as well, though the survival of all other cancers among females remained similar or decreased.
Table II. Five-year relative survival rates of AYAs with cancer by age and gender.
Second primary cancers
Overall, with a median follow-up time of 6.8 years, 884 patients (3.8% of all AYAs) were diagnosed with at least one second primary cancer. In 105 of these 884 patients (11.9%), the second tumor occurred within three months after diagnosis of the first tumor. A large part of the remaining 779 patients with non-simultaneous second primary cancers consists of melanoma, contra-lateral testicular cancer, and contra-lateral breast cancer. With the application of the IARC/IACR rules 412 AYAs (1.8% of all AYAs) are defined to have a second primary tumor. In the SIRs are presented for all primary sites combined. Male AYAs diagnosed with cancer have a more than six times increased risk of developing a second primary cancer. After application of the IACR/ IARC rules, this risk is still more than three times increased. For females, the risk of developing a second primary cancer is almost five- and two times increased, respectively. Overall, melanoma is the most frequently diagnosed second primary cancer among AYAs. The risk of developing a melanoma after a first primary cancer was six times increased for males and eight times increased for females. This risk was even higher in patients with a melanoma as first primary cancer (females SIR: 22.8 and males SIR: 27.2, not shown in ). After application of the IARC/IACR rules for multiple cancers, the most frequently diagnosed second primary cancers in males were hematological malignancies (n = 40, SIR: 3.6, 95% CI 2.6–4.9), gastrointestinal tumors (n = 32, SIR: 5.2, 95% CI 3.6–7.4) and gonadal germ cell tumors (n = 28, SIR: 1.9, 95% CI 1.2–2.7). For females these second primary cancers included breast cancer (n = 72, SIR: 1.8, 95% CI 1.4–2.2), melanoma (n = 25, SIR: 1.2, 95% CI 0.8–4.5) and hematological malignancies (n = 23, SIR: 2.6, 95% CI 1.8–4.0). We also explored the risks of second primary cancers after tumor-specific first primary cancers (data not shown). The most striking result was the more than seven times increased risk of breast cancer after Hodgkin's disease (n = 26, SIR: 7.4, 95% CI 4.8–10.8).
Table III. Standardized Incidence Ratios of second primary cancers after a first primary cancer in AYAs.
Discussion
Recent, population-based data about cancer among AYAs is scarce. This study was performed to provide insight in cancer incidence, survival and risk of second primary cancers among AYAs in the Netherlands. This information is necessary to define the extent and nature of the AYA patient population and provides input for further research to improve quality of care in this specific group of patients. Similar to earlier findings, the incidence of cancer in AYAs has increased significantly between 1989 and 2009 in the Netherlands [Citation1–4]. A comparison with incidence data from the SEER registry shows a slightly higher cancer incidence among AYAs in the US (http://seer.cancer.gov/) [Citation1]. The most prominent observed trend over time in male AYAs concerned testicular cancer, where the incidence has more than doubled in the period 1989–2009. This increase in testicular cancer has been reported worldwide [Citation11]. An accumulating body of evidence suggests that testicular cancer may originate already during fetal life, possibly associated with impaired spermatogenesis, cryptorchidism and hypospadias. As a common causal factor for this combination (also called Testicular Dysgenesis Syndrome) exposure to endocrine disrupting chemicals has been postulated [Citation12]. In female AYAs a moderate increase in incidence over time was found for melanoma and breast cancer. A steep increase in melanoma incidence is seen among adult females (and males) as well, which is probably largely due to a combination of more UV exposure (sun bathing and sunbed use) and increased awareness [Citation13,Citation14]. The same explanation might be underlying the observed rise in melanoma in female AYAs. A recent study performed in Australia, including patients with melanoma between 18 and 39 years old, found that sunbed use during adolescence and early adulthood was associated with increased risk of early onset melanoma [Citation15]. No obvious explanation is available for the observed increase in breast cancer over time in AYAs. The known lifestyle factors associated with breast cancer risk such as age at birth of first child, number of pregnancies, hormonal therapy, breast feeding are in these AYAs not very likely causes, as in our analyses only women younger than 30 years were included. Furthermore, a decreasing trend in astrocytomas is observed. This was also reported by Bleyer and colleagues [Citation1] but no clear explanation is available. Next to variation in incidence over time, considerable variation exists in the incidence of specific cancer types across the AYA continuum. Within the male AYA population, the gonadal germ cell tumors are the most frequently diagnosed cancer at each age, but the proportion of these tumors increased strongly with increasing age (from 19% to 35%). In the youngest males, Hodgkin's disease, non-Hodgkin lymphoma and ALL jointly represent over 30% but this proportion decreased towards the older age groups. Melanoma, on the other hand, showed a clear increase with increasing age. Similar to males, in young female AYAs Hodgkin's disease and non-Hodgkin's lymphoma represented the majority of diagnosed cancers but with increasing age, the predominant cancers become melanoma and breast cancer. Similar patterns are reported in the US by Bleyer and colleagues [Citation1].
This study showed a typical distribution of cancer types among AYAs, very different from the pattern reported for children and older adults. Frequently occurring cancers during childhood such as neuroblastoma, nephroblastoma (Wilm's tumor), other embryonal tumors and retinoblastoma are uncommon among AYAs. ALL is the most common hematological malignancy among children [Citation5]. The different types of leukemia in AYAs reflect a clear transition of a childhood pattern, with ALL as most frequently diagnosed in the AYAs aged 15–19 years, to an adult pattern dominated by AML and rising incidence of CML. Concerning soft tissue and bone cancers a shift is seen as well; younger AYAs are more frequently diagnosed with ‘childhood and adolescent types’ such as osteosarcoma, Ewing's tumor and rhabdomyosarcoma whereas older AYAs are diagnosed with other types of soft tissue and bone cancers. Non-Hodgkin lymphomas are diagnosed in children, AYAs and adults, but a different distribution of histological subtypes has been reported [Citation16]. Frequently diagnosed carcinomas in adults such as colorectal, lung and prostate cancer are very rare among children and AYAs [Citation8], with the exception of breast cancer and melanoma in females. However, the underlying etiology might be different in AYAs compared to older women.
The overall five-year relative survival of AYAs diagnosed with cancer in the Netherlands is 80–82%. This is better compared to survival rates for AYAs in the US (US female AYAs have a similar survival but US male AYAs have a five-year survival of approximately 70% [Citation1]), but these figures relate to a more distant time period (1975–1999). The EUROCARE study reported a five-year survival rate of 87% for patients diagnosed at age 15–24 years [Citation6]. Time trends were studied as well in EUROCARE and a small improvement in the five-year survival rate in the period 1995–1999 versus 1990–1994 was reported. In our study, we observed an improvement in the five-year survival in males as well as females since 1989, from 74% to 86% in males and from 79% to 86% in females. Part of the survival improvement in males might be caused by the increased proportion of patients with testicular cancer which have a very good prognosis. Therefore, survival over time was assessed after exclusion of these tumors as well. A similar trend and improvement in survival was observed (five- year survival of 67% in the period 1989–1993 and 79% in the period 2004–2009). It can be concluded that the progress made in survival is not due to the strongly increased number of patients with testicular cancer. The most prominent survival improvement is seen in patients with non-Hodgkin lymphoma. This is in line with findings from EUROCARE in which a significant increased survival was reported in these patients as well [Citation6]. In general the five-year survival rates of the most frequently diagnosed tumors in male AYAs seem to improve over time. As the number of patients by tumor site is relatively small, observed trends should be interpreted with care. During the study period there might have been influence of changing clinical practice, with more centralization and more patients discussed in multidisciplinary teams and a better adherence to tumor directed guidelines. However whether these factors have played a role in our study population is unclear as literature on this topic in AYA cancers is very scarce. Also, stage migration over time might have had a positive effect on survival. An elaborate discussion on stage migration was beyond the scope of this overview paper. In female AYAs time trends by tumor site are less clear; next to non-Hodgkin lymphoma, only survival of breast cancer and Hodgkin's disease patients has clearly improved over time. In the most recent period the survival of AYAs with breast cancer seems similar compared with the survival of adult breast cancer patients (five-year survival of 84% vs. 86%) (http://www.cancerregistry.nl). The survival improvement seen in young breast cancer patients was also observed in other European countries [Citation6]. However, a US study based on SEER data reported five-year survival rates of young breast cancer patients which were less than 80% and stated that improvement over time was less compared to the adult breast cancer population, probably because of age-dependent biological differences [Citation17].
A relatively poor survival is noted among AYAs diagnosed recently with ALL (five-year survival of 50–52%), AML (five-year survival of 44–48%), astrocytoma (five-year survival of 54–57%), rhabdomyosarcoma (five-year survival of 36–39%), Ewing's tumor (five-year survival of 40–46%) and osteosarcoma among males (five-year survival of 45%). The survival of these cancers in children (0–14 years) in the Netherlands is much better: Children with ALL have a five-year survival of approximately 80%, with AML 53%, with astrocytoma 76%, with rhabdomyosarcoma 65%, with Ewing's tumor 65% and with osteosarcoma 63% (unpublished data from the Netherlands Cancer Registry, 2003–2007). Similar survival rates in children were reported by Linabery and Ross (2008) [Citation18] and by the EUROCARE study [Citation6]. The poor survival among AYAs with these cancers which are also prevalent among children may be partly explained by the low participation rate of AYAs in clinical trials [Citation19,Citation20] but tumor biology may be different as well [Citation21]. Another explanation might be that AYAs are diagnosed at a more advanced disease stage due to patient's and doctor's delay [Citation22]. There is an ongoing debate about the question whether to treat AYAs with cancer as children or as adults [Citation23]. In case of ALL, adolescents have better survival in case they are treated with pediatric rather than adult protocols [Citation24].
In this study we had to use relative survival as an approximation for disease-specific survival because disease-specific survival cannot be estimated as no information on the cause of death was available to the Netherlands Cancer Registry due to specific legislation concerning Statistics Netherlands, holder of information on causes of death in the Netherlands. This relative survival adjusts for the general survival of the Dutch population taking gender, age, and calendar year into account. This might be interpreted as a limitation of this study. However, it can be argued that relative survival is the most appropriate method to use in population-based cancer survival studies, as misclassification of cancer specific deaths, results in biased estimates for cancer-specific survival [Citation25]. Furthermore, it should be noted that Death Certificate Only (DCO) registrations are not available in the Netherlands Cancer Registry due to the same reason as mentioned before. These patients would not affect the survival results as they would be excluded from the survival analyses.
Next to incidence and survival, we assessed the risk of second primary cancers in this study. A striking result was that AYAs with cancer have a more than six times (in case of male AYAs) or almost five (in case of females) increased risk to develop a second primary cancer. A large part of these second primary cancers are bilateral or of the same type (breast cancer, testicular cancer and melanoma). But also after excluding these bilateral/same-type-tumors, the risk of developing a second primary was approximately three times increased in males and two times in females. Although in the current study the mean follow-up time was almost 10 years and the median follow-up time was almost seven years, part of the second primary cancers may occur later in life. The reported risk of second primary cancers may therefore be an underestimation. Several explanations can be given for the increased risk of second primary cancers: patients could be genetically susceptible for (different types of) cancer; environmental factors early in life may account for both the first and the second tumor; the second primary cancers may be treatment-induced; and/or general survival improvement of the first primary cancer may result in more second primary cancers. Compared to the earlier performed Dutch study from van Gaal et al. [Citation7], the reported risk on second primary cancers is much lower (i.e. 5–6-fold increased risk vs. 31-fold). Our data are more recent and much more precise due to a 20 year national coverage of all AYA tumors in The Netherlands. However, it should be mentioned that due to fact that the NCR is complete since 1989, first primary tumors prior to 1989 may not be recorded and therefore, secondary primary tumors may not be identified as such. Although, all available information before 1989 was incorporated to identify first primary cancers, this could have led to an underestimation of the risk of secondary primary cancers.
As far as we know no studies have been performed in this age group evaluating the risk of second primary cancers. Studies including children with cancer reported on average a six-fold increased risk of second primary cancers [Citation26,Citation27].
In conclusion, this study demonstrates that the incidence of cancer at AYA age is rising. In general, the survival has improved over time, but for specific tumor sites survival still lacks behind compared to children. The risk of second primary cancers is high and might even increase in the future as a result of survival progress. Cancer specific studies are needed to explain these unique features and to translate the figures into useful prognostic information for patients. Specialized follow-up of all AYAs after treatment of the primary tumor is needed to diagnose second primary cancers in a stage where cure can still be reached. Finally, tailor made treatment regimes should be developed for this age group as AYAs with cancer live in a completely different psycho-social context compared to older cancer patients, with specific dynamics in education, careers, social networks, sexual relations and family life.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bleyer A, O’Leary M, Barr R, Ries LAG. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER Incidence and survival 1975–2000. Bethesda, MD: National Cancer Institute, NIKH Pub. No. 06-5767; 2006.
- Croucher C, Whelan JS, Moller H, Davies EA. Trends in the incidence and survival of cancer in teenagers and young adults: Regional analysis for South East England 1960–2002. Clin Oncol (R Coll Radiol) 2009;21: 417–24.
- van der Horst M, Winther JF, Olsen JH. Cancer incidence in the age range 0–34 years: Historical and actual status in Denmark. Int J Cancer 2006;118:2816–26.
- Stiller CA, Desandes E, Danon SE, Izarzugaza I, Ratiu A, Vassileva-Valerianova Z, . Cancer incidence and survival in European adolescents (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2006–18.
- Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev 2010;36:277–85.
- Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, . Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer 2009;45:992–1005.
- van Gaal JC, Bastiaannet E, Schaapveld M, Otter R, Kluin-Nelemans JC, de Bont ES, . Cancer in adolescents and young adults in north Netherlands (1989–2003): Increased incidence, stable survival and high incidence of second primary tumours. Ann Oncol 2009;20:365–73.
- Birch JM, Alston RD, Kelsey AM, Quinn MJ, Babb P, McNally RJ. Classification and incidence of cancers in adolescents and young adults in England 1979–1997. Br J Cancer 2002;87:1267–74.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- IARC. International rules for multiple primary cancer ICD-O, 3rd edition. Internal report no. 2004/02. Lyon: IARC; 2004.
- Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev 2010;19:1151–9.
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil Steril 2008;89(2 Suppl):e33–8.
- Holterhues C, Vries E, Louwman MW, Koljenovic S, Nijsten T. Incidence and trends of cutaneous malignancies in the Netherlands, 1989–2005. J Invest Dermatol 2010; 130:1807–12.
- Levell NJ, Beattie CC, Shuster S, Greenberg DC. Melanoma epidemic: A midsummer night's dream? Br J Dermatol 2009;161:630–4.
- Cust AE, Armstrong BK, Goumas C, Jenkins MA, Schmid H, Hopper JL, . Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer 2011;128:2425–35.
- Sant M, Allemani C, Tereanu C, De AR, Capocaccia R, Visser O, . Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood 2010;116:3724–34.
- Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol 2009;36:237–49.
- Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008;113:2575–96.
- Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials – international comparisons, barriers, and implications. Semin Oncol 2010;37:e1–8.
- Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer 2008;50(5 Suppl):1101–4.
- Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 2008;8:288–98.
- Dang-Tan T, Trottier H, Mery LS, Morrison HI, Barr RD, Greenberg ML, . Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatr Blood Cancer 2008;51:468–74.
- Eden T. Keynote comment: Challenges of teenage and young-adult oncology. Lancet Oncol 2006;7:612–3.
- de Bont JM, Holt B, Dekker AW, van der Does-van den Berg, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs. adult protocols in the Netherlands. Leukemia 2004;18:2032–5.
- Sarfati D, Blakely T, Pearce N. Measuring cancer survival in populations: Relative survival vs. cancer-specific survival. Int J Epidemiol 2010;39:598–610.
- Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, . Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011;305:2311–9.
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, . Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083–95.