Abstract
Aim. To present a retrospective analysis of results of definitive radiotherapy for rectal cancer. Material and methods. Forty-one consecutive patients with rectal cancer (32% primary, 61% pelvic recurrence and 7% after R2 resection) who could not be treated with surgery underwent external beam radiotherapy. A median tumour dose of 64 Gy was given with 1.8–2.5 Gy per fraction using 2D or 3D technique. In 46% of patients, concurrent 5-Fu-based chemotherapy was given. The median follow-up was 54 months. Results. Clinical complete response was achieved in 39% of patients. Five-year cumulative incidence of local failure, overall survival and cancer specific survival were 76%, 26% and 30%, respectively. Of 11 patients with local control, in five cases the tumour was larger than 5 cm and in the other five the tumour was fixed. Two patients, regarded as locally controlled had non-progressive tumour without local symptoms at the last follow-up of 54 and 118 months post-radiotherapy. Late toxicity occurred in 22% of patients, all with acceptable severity. There was no bowel obstruction requiring surgery despite that in 18 patients the small bowel dose was > 60 Gy to a mean volume of 51 cm3. Conclusion. Definitive radio(chemo)therapy provides a chance for local control even in patients with large fixed or recurrent rectal cancer.
Transabdominal surgery alone or combined with pre-operative radiotherapy (or radiochemotherapy) is the mainstay of treatment for rectal tumours. However, there is a small group of patients who cannot be operated due to co-morbidity or patients’ refusal. In patients with small superficial tumour, full thickness local excision or orthovoltage intraluminal contact x-ray therapy or brachytherapy are valuable treatment options [Citation1,Citation2]. However, in patients with deeply infiltrating lesions, such methods cannot be employed due to the technical limitation of local excision or inadequate dose distribution within the target volume using contact x-ray therapy or brachytherapy. The use of definitive external beam radiotherapy has been reported in those patients [Citation3–13]. However, most reports have been published prior to the conformal radiotherapy era, thus the irradiation dose has been limited to less than 60 Gy. In patients with large fixed tumour the outcome was poor, so only palliative treatment was recommended [Citation8].
As dose-response relationship in rectal adenocarcinomas has been demonstrated [Citation2,Citation5,Citation13], we postulate that an increase of total dose may result in an increased chance for local cure even in patients with large fixed lesions. Further support for this concept has been provided by studies showing that dose escalation up to 60 Gy or more is feasible [Citation3,Citation9,Citation10]. It is also probable that further improvement can be obtained by adding concurrent chemotherapy to radiation because such combination has yielded superior local control compared to radiation alone in a preoperative setting [Citation15,Citation16]. In addition, it has also been demonstrated that in large fixed tumours, preoperative chemoradiation leads to pathological complete response in 16% of patients [Citation16]. Furthermore, it has been reported that meaningful proportion of patients with clinical complete response (cCR) after chemo-radiation can be cured without surgery [Citation17].
For the above reasons, since the year 2000 a radical external beam radiotherapy using doses higher than 60 Gy was administered in our patients with advanced rectal cancer. In order to deliver such high doses, in some patients the recommended 50 Gy limit for small bowel tolerance [Citation18] had to be violated. Given the paucity of published data, a retrospective study was undertaken in order to present the local effectiveness and to evaluate the toxicity of such an approach. In particular, we aimed at answering the questions whether local cure is possible in patients with large fixed lesion and whether doses higher than 50 Gy can be safely delivered to the limited volume of small bowel.
Material and methods
Inclusion criteria for the current analysis consisted of definitive radical external beam radiation alone or in combination with chemotherapy given between January 2000 and January 2008 in patients with primary or recurrent rectal adenocarcinoma. Patients needed to be treated with radical intent, no surgery after radiotherapy was planned, no distant metastases were present and there was no previous pelvic radiotherapy. The review of database identified 41 consecutive patients who fulfilled the entry criteria. These patients constituted 2.3% of the total of 1788 patients with rectal cancer who received radical radiotherapy (preoperative, postoperative or definitive) at that time in our department. The work-up included clinical examination, rectoscopy or colonoscopy, pelvic computed tomography (CT), chest x-ray, abdominal ultrasound or CT, blood count, blood biochemistry, and carcinoembryonic antigen (CEA) serum level. Patients’ characteristics are listed in . There were 13 patients (32%) with primary tumour, 25 patients (61%) with pelvic recurrence after surgery and three patients (7%) who underwent R2 resection. We included three patients after R2 resection because tumours left behind after surgery were visible on the post-operative CT scan. Nineteen patients (48%) had advanced tumour measuring 5 cm or more in the greatest dimension on pelvic CT. In 20 patients (59%), the tumour was fixed on digital rectal examination (). In all patients, the decision about using radical radiotherapy without surgery was made at the multidisciplinary meetings. Extensive en bloc resection of involved adjacent organs or structures was always considered. The tumour was considered as unresectable when it was invaded or abutted to structures not possible to resect even after preoperative radiotherapy, e.g. infiltration into S1-2. Reasons for ruling out surgical treatment are shown in . Rectal adenocarcinoma was pathologically confirmed in 32 patients (78%). In the remaining nine patients (22%), where all of them had pelvic recurrence, previously treated primary tumour was pathologically confirmed, but local relapse was not. In these patients, diagnosis of pelvic recurrence was based on the presence of a tumour on pelvic CT interpreted by radiologist as recurrence combined either with increase of local symptoms or increase of pelvic tumour size in pelvic CT.
Table I. Characteristics of patients.
There were no predefined written guidelines describing technique for definitive radiotherapy of rectal cancer. In order to reduce toxicity, in six frail patients (15%) only gross tumour volume with a margin was treated using 3-dimensional (3D) technique. In the remaining 35 (85%) patients, large elective pelvic volume and then a boost volume that included only gross lesions were irradiated. Simulator based 2-dimensional (2D) three-field technique (two laterals and one posterior) was used to irradiate elective area. Cranial border of all fields was placed 1 cm above sacral promontory. Caudal border was 1 cm below anorectal ring or 2 cm below the tumour for very low-lying lesions. The lateral borders of posterior fields extended 1–1.5 cm beyond the pelvic brim. Anterior border of the lateral fields was located on the inner surface of pubic symphysis and the posterior border 1 cm beyond the inner surface of the sacral bone. Doses were prescribed to the intersection of the axes of the fields. The elective dose varied between 42.5 and 50.4 Gy given with 1.8–2.5 Gy per fraction (most patients received 2 Gy). Next, the boost dose was added using 3D technique in 29 patients and 2D technique in the remaining six patients. Target volume for a boost consisted of gross tumour with whole circumference of the rectal wall at tumour level with 1–2 cm margin in case of the treatment of primary rectal cancer or anastomotic recurrence and gross tumour volume with 1 cm margin in case of other types of recurrence. This margin was trimmed to 0.5 cm within small bowel volume. In the construction of the treatment plan, critical structures were small bowel, urinary bladder, femoral bones and sacral canal. No predefined dose-volume constraints were used for these organs at risk. Doses of more than 50 Gy were allowed to a limited volume of small bowel. The amount of small bowel within a boost volume influenced the decision regarding the choice of the boost dose. The boost dose distribution had to be kept within 95–107%. The boost dose was delivered using 2 Gy or 2.5 Gy per fraction. Treatment was given with 15 MV x-ray (N = 34), 6 MV x-ray (N = 2) or Co-60 (N = 5). Multileaf collimators or customised blocks were used to shield the organ at risk. Patients were advised to maintain full urinary bladder during CT done for treatment planning and during irradiation. Irradiation was performed on a belly-board. The total dose varied from 55 Gy to 70 Gy, median 64 Gy. Biologically equivalent doses to fractionation given with 2 Gy per fraction varied from 58 Gy to 76.25 Gy with median value of 64 Gy. All patients but one received equal or more than 60 Gy of equivalent dose. Equivalent doses were calculated using the linear-quadratic model and the following equation; biologically equivalent dose = nd (d + α/β)/(2 Gy + α/β); where n = number of fractions, d = dose (Gy) per fraction and α/β = 3 Gy for late damage.
In 19 patients (46%) concurrent three two weeks cycles of chemotherapy were planned. Each cycle consisted of bolus 5-fluorouracil 400 mg/m2 and leucovorin 20 mg/m2 per day given on Days 1 and 2 of each cycle. One patient received neoadjuvant chemotherapy. The remaining 21 (51%) patients were unfit for chemotherapy. Adjuvant chemotherapy was given to two patients.
Patients were followed at three-month intervals. Evaluation consisted of physical examination and CEA serum level. Pelvic CT and other examinations were performed if treatment failure was clinically suspected, or in case of an increased CEA level. Occasionally, pelvic CT was also done for asymptomatic patients with normal CEA. cCR was defined as no tumour felt on digital rectal examination or as no tumour seen on rectoscopy in patients with cancer which was initially located in high rectum. Local failure was defined as tumour re-growth after cCR or as an increased size of persistent pelvic tumour detected either by clinical examination or pelvic CT or as a progression of local symptoms in patients with stable persistent tumour. Pathological verification of treatment failure was not routinely performed. Biopsies were taken in only two patients showing viable cancer. Local recurrences were recorded irrespective of distant metastases occurrence. Local control was defined as sustained cCR at last follow-up or as a long-lasting residual mass seen on pelvic CT but without increase in size or progression of clinical symptoms. The follow-up time for all patients ranged from 5 to 118 months with median of 28 months and for nine living patients from 37 to 118 months with median of 54 months. The vital status was known in all patients. One patient was lost to follow-up after completion of treatment and died after three months. This patient was regarded as having local tumour progression. Late radiation damage was recorded in locally controlled patients or in patients with local recurrence when symptoms were interpreted as caused due to post-radiation damage. Acute and late complications were scored according to the RTOG-EORTC scale [Citation19].
For the purpose of this study, in 32 of patients (78%), planning CT scans were retrieved from our database in order to evaluate the association between dose-volume parameters and the incidence of late radiation damage. For three of the remaining nine patients the planning CT was not available and six were treated with 2D technique. Elective fields were reconstructed in the 3D-planning system in order to obtain the 3D-dose distribution in electively treated volume and next the cumulative 3D-dose distribution of both elective and boost phases of treatment was displayed and analysed. The median volume of electively treated area within 90% isodose was 2251 cm3, ranging from 1060 to 4890 cm3. The median volume of planning target volume (PTV) of boost or PTV in patients treated without irradiation of elective area was 352 cm3, ranging from 82 to 1606 cm3. The small bowel and urinary bladder were contoured. Each of the small bowel loops were contoured separately. Contouring of the critical structures was performed independently by two radiation oncologists. Next, the differences in delineation were visualised and consensus contours were outlined. Dose-volume histograms for small bowel and urinary bladder were created.
Two-sided χ2-test or Fisher's exact test was used to compare proportions. The Kaplan-Meier method was used to calculate survival and cumulative incidence of local failure. In the calculation of cancer specific survival, the event was death with cancer. Patients without local or distant disease were censored at the time of death from non-rectal-cancer cause or at the time of last follow-up. In the calculation of the cumulative incidence of local failure, patients free of local failure were censored at the time of death from non-rectal-cancer cause or death from distant metastases or when they were alive at last follow-up. All time intervals were measured from the first day of radiotherapy. The data was analysed with SPSS version 19 for Windows (SPSS, Chicago, Illinois, USA).
Results
All patients received planned radiation dose. Two patients did not receive the last course of chemotherapy due to leucopoenia. Skin, intestinal or genitourinary grade III-IV acute toxicity was observed in 11 patients (27%; 95% confidence interval [CI] 13–41%). There was only one patient with grade IV toxicity; he had severe haematuria requiring hospitalisation and blood transfusion.
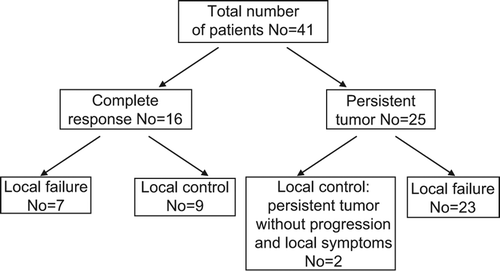
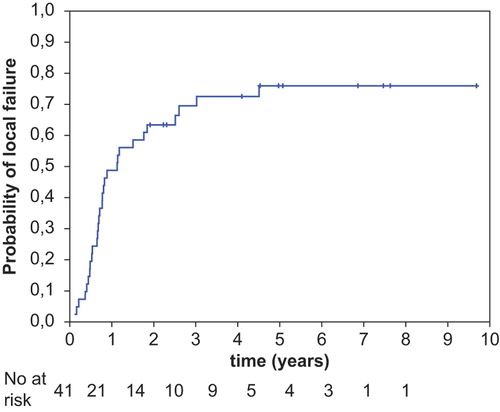
cCR was achieved in 16 patients (39%, 95% CI 24–54%) and was confirmed by CT scan in 12 of them. In the remaining four patients cCR was diagnosed based on digital rectal examination. cCR was observed in seven patients at the first follow-up visit two to three months post-radiotherapy and in the remaining nine patients between three and 10 months post-radiotherapy. Median time between treatment completion and cCR diagnosis was four months. Of 16 patients with cCR, nine (56%) had local control and in seven (44%) cancer recurred locally. In total, local control was observed in 11 patients (27%) (). Of these, two patients had non-progressive residual lesion seen on pelvic CT without local symptoms and an increase of CEA level at last follow-up 54 and 118 months post radiotherapy. Local control was confirmed by the pelvic CT in 10 patients and in remaining one by colonoscopy. Local effectiveness in relation to the pre-treatment patients’ characteristics is presented in . Local control was achieved in four of 13 patients (31%) with primary tumour, in six of 25 patients (24%) with local recurrence after previous surgery and in one of three patients after R2 resection. Of 11 patients with local control, in five of them, initial maximal tumour dimension was equal to or larger than 5 cm and in five the tumour was fixed. Of 21 patients who were treated with radiation alone, local control was recorded in four (19%). Of 20 patients who were treated with radiation and chemotherapy, local control was recorded in seven (35%). Local failure was detected in 30 patients (73%). In all these patients, the recurrence was located within pretreatment boost area. Five-year cumulative incidence of local failure was 76% (95% CI 62–90%) (). In 22 patients, local failure was the only site of recurrence and the remaining eight patients also had distant metastases. In two patients distant metastases were detected alone.
Table II. Local control in relation to the pre-treatment patients’ characteristics.
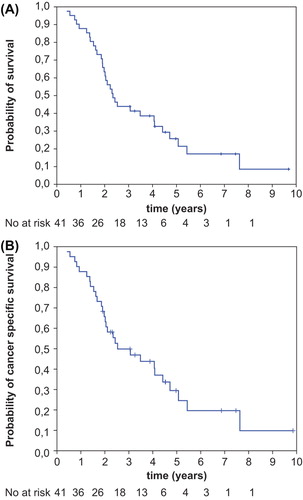
Thirty-two patients (78%) died, of whom 29 patients had cancer and three patients free from cancer died due to the intercurrent disease. Nine patients (22%) were alive from 37 months to 118 months after treatment, with median value of 54 months. Of these, three had local failure and six were free from cancer, of whom one had R2 resection and five were treated due to the pelvic recurrence. Five-year overall survival was 26% (95% CI 11–41%) and five-year cancer specific survival was 30% (95% CI 14–45%) ().
Overall 13 late complications (12 grade II and one grade III) occurred in nine patients (22%); seven of these patients had the local disease controlled and two patients had local recurrence. Five patients suffered from rectal bleeding requiring one procedure of laser coagulation; three patients also had one procedure of blood transfusion. Four patients developed bowel obstruction which was treated conservatively with bowel rest; one patient needed several hospitalisations for multiple episodes of bowel obstruction. All patients with bowel obstruction had previous pelvic surgery. One patient developed stenosis of anastomosis and pelvic abscess requiring stoma creation. Two patients developed urinary incontinence. One patient developed skin and vaginal fibrosis. There were no patients manifesting late chronic diarrhoea, malabsorption or late urinary bladder radiation toxicity. Twenty-one patients received doses to the urinary bladder higher than 60 Gy to the mean volume of 35 cm3, ranged from 1 to 105 cm3; 10 patients received doses higher than 65 Gy to the mean volume of 19 cm3, ranged from 1 to 49 cm3.
We did not observe the apparent association between dose-volume parameters and late small bowel complications (). Of note, there was no bowel obstruction requiring surgery even though in 18 patients the small bowel dose was higher than 60 Gy within the mean volume of 51 cm3. The median follow-up in all of these 18 patients was 24 months, ranging from 5 to 92 months.
Table III. Late postradiation small bowel damage in relation to dose and volume in 32 patients for whom 3D dose distribution within small bowel was available.
Discussion
Our results demonstrated that high dose radiotherapy for unresected rectal cancer results in local control in low but clinically meaningful proportion (27%) of patients. In the subgroup of patients achieving cCR, the local control was 56%. Previous opinion [Citation8] that only palliation can be offered for patients with large fixed lesions may be questioned as in the current series, the local control was achieved in some of such cases.
To our knowledge, there is no data in literature presenting late adverse effects in patients with rectal cancer receiving total dose of more than 60 Gy using high-dose external beam x-ray radiation. That risk observed in the current series seems to be acceptable. However, due to the short observation periods () related mostly to the high death rate caused by poor effectiveness of treatment, these data should be interpreted with caution. Hence, they may not be generalised to other patient populations. There was no small bowel obstruction requiring surgery in our patients. Similarly, no severe late small bowel toxicity was recorded in a cohort of 17 patients with retroperitoneal neoplasms who underwent proton therapy and received 60 Gy or more to the small volume (mean 7.7 cm3, range 0.37–29 cm3) of small bowel [Citation20]. In contrast, Glanzmann [Citation21] reported 13% (6 of 47) of severe or fatal late small bowel complications after dose higher than 60 Gy in old series of patients with uterus or bladder cancer. The mean small bowel volume for all his patient material was estimated to be 282 cm3 [Citation22]. Gallagher et al. [Citation23] reported 30% (6 of 20) of small bowel complications in patients who received small bowel doses between 55 and 65 Gy using 2D technique. In this series, an average small bowel volume receiving more than 55 Gy was 14 cm3 in patients without complications and 317 cm3 in patients with small bowel obstruction. In the old series, severe late small bowel complications rose to approximately 50% when 60 Gy was given in large volumes using two opposed parallel anterior–posterior fields [Citation24,Citation25]. As the risk of injury is strongly related to the volume of small bowel receiving high dose [Citation22,Citation23,Citation26], small amount of organ within high dose region explains low risk of complication in the current series. Because of bowel mobility, it is possible that the received dose to small bowel loops is lower than what is estimated by dose-volume histograms.
Our data confirmed previously published observation that rectal cancer regression after radiotherapy is slow and may last up to 11 months until complete response is achieved [Citation8]. Two of our patients had long-lasting non-progressing residual pelvic mass after 54 and 118 months from treatment. Therefore, persistence of asymptomatic tumours that did not increase in size in patients without increase of CEA level should not be regarded as a treatment failure. Such observation has not been previously reported in the literature in patients with rectal cancer.
There is limited retrospective data on the role of definitive external beam radiotherapy in primary or recurrent rectal cancer [Citation3–13,Citation27–29]. In most studies, treatment was based on old 2D radiotherapy technique usually with the total dose delivered of less than 60 Gy. The largest series of 271 patients with primary tumour was published by Wang et al. [Citation8]. The total dose delivered using 2D technique varied from 40 Gy in 15 fractions given over three weeks to 60 Gy in 30 fractions given over six weeks. Most frequently prescribed dose was 52 Gy in 20 fractions over four weeks. The median follow-up was 40 months. cCR was achieved in 30% of patients. Of these, local relapse was observed in 78%. There was no information about whether there was any long-lasting local control in patients with persistent tumour. Some patients underwent rescue surgery. Five-year and 10-year overall survival was 27% and 10%, respectively. In other smaller studies with the use of total doses of equal or less than 60 Gy, cCR rate varied between 29% and 56%, crude rate of local control between 8% and 73% and five-year survival between 0% and 60% [Citation4–7,Citation11–13,Citation22]. Those differences in outcome can be explained by a variation in case mix, duration of follow-up, number of patients lost to follow-up and the use of rescue surgery. Favourable predictors for tumour control were small tumour size, its mobility and cCR [Citation4,Citation5,Citation7,Citation8].
We have found only three studies adequately reporting results after irradiation of rectal cancer with median total doses of more than 60 Gy. Overgaard et al. [Citation10] reported results for 55 patients receiving definitive radiotherapy or radiochemotherapy with total doses ranging from 50 Gy to 70 Gy given in 25–30 fractions using 2D technique. cCR was observed in eight patients (15%); five of them survived for more than three years after radiation and had local disease controlled. Frykholm et al. [Citation3] described results of 11 patients with pelvic recurrence after surgery who were then treated with definitive radical radiotherapy using physical total dose or biologically equivalent total dose of more than 60 Gy up to 66 Gy either alone or combined with misonidazole or chemotherapy. Three patients were locally symptom-free at last follow-up and two patients survived for five years. In the abstract, Yamada et al. [Citation9] presented results from carbon ion therapy in 90 patients with local recurrence after surgery. The total dose ranged from 67.2 GyE to 73.6 GyE. The five-year local control was 81% and five-year overall survival was 43%. The data regarding late adverse effects was not provided in all of the above three reports.
Recently a “wait and see” policy has been widely discussed for patients who achieved cCR about two months after chemoradiation [Citation17,Citation29]. Our data do not support such approach for patients with advanced primary or recurrent cancer for two reasons. Firstly, local control was observed only in approximately half of complete responders. Similar finding was recently published by Hughes et al. [Citation29]. Secondly, majority of cCR were manifested later than two months after chemoradiation, usually after three to 10 months.
The weakness of our evaluation is that it is retrospective in nature. The risk of late adverse effects may be underestimated due to the high local recurrence rate.
In order to improve results, a further dose escalation can be provided by adding an extra boost using endocavitary contact x-ray radiotherapy or brachytherapy after the external beam radiation if residual lesion is small [Citation2,Citation14,Citation27,Citation30]. Another possibility is to increase an external beam radiation boost dose by reducing the margin around gross tumour volume with image guided technique using radio-opaque markers for tumour localisation. Combination of radiation with hyperthermia [Citation31] or using carbon ion radiotherapy [Citation9] is another option that could be considered for tumour control improvement.
In conclusion, high dose definitive external beam radiation results in local control and long-term survival in a meaningful proportion of patients with rectal cancer even in those with large fixed tumour.
Acknowledgements
Presented in part at 27th Meeting of European Society for Therapeutic Radiology and Oncology, Sweden, Goteborg, September 2008. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 2009; 96:280–90.
- Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: The Lyon R96-02 randomized trial. J Clin Oncol 2004; 22:2404–9.
- Frykholm GJ, Påhlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol 1995;34:185–94.
- Lim L, Chao M, Shapiro J, Millar JL, Kipp D, Rezo A, et al. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum 2007;50:2032–9.
- Overgaard M, Overgaard J, Sell A. Dose-response relationship for radiation therapy of recurrent, residual, and primarily inoperable rectal cancer. Radiother Oncol 1984; 1:217–25.
- Rades D, Kuhn H, Schultze J, Homann N, Brandenburg B, Schulte R, et al. Prognostic factors affecting locally recurrent rectal cancer and clinical significance of hemoglobin. Int J Radiat Oncol Biol Phys 2008;70:1087–93.
- Taylor RE, Kerr GR, Arnott SJ. External beam radiotherapy for rectal adenocarcinoma. Br J Surg 1987;74:455–9.
- Wang Y, Cummings B, Catton P, Dawson L, Kim J, Ringash J, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: Long term outcome of 271 patients. Radiother Oncol 2005;77:126–32.
- Yamada S, Yanagi T, Hara R, et al. Carbon ion therapy for patients with locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2008;72(Suppl):131. [Abstract].
- Overgaard M, Bertelsen K, Dalmark M, Gadeberg CC, von der Maase H, Overgaard J, et al. A randomized feasibility study evaluating the effect of radiotherapy alone or combined with 5-fluorouracil in the treatment of locally recurrent or inoperable colorectal carcinoma. Acta Oncol 1993;32:547–53.
- Rominger CJ, Gunderson LL, Gelber RD, Conner N. Radiation therapy alone or in combination with chemotherapy in the treatment of residual or inoperable carcinoma of the rectum and rectosigmoid or pelvic recurrence following colorectal surgery. Am J Clin Oncol 1985;8:118–27.
- Wong CS, Cummings BJ, Keane TJ, O’Sullivan B, Catton CN. Results of external beam irradiation for rectal carcinomas locally recurrent after local excision or electrocoagulation. Radiother Oncol 1991;22:145–8.
- Lybeert ML, Martijn H, de Neve W, Crommelin MA, Ribot JG. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery: Definite but limited influence on relapse-free survival and survival. Int J Radiat Oncol Biol Phys 1992;24:241–6.
- Gerard JP, Chapet O, Ortholan C, Benezery K, Barbet N, Romestaing P. French experience with contact X-ray endocavitary radiation for early rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:661–73.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemoradiotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23.
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26: 3687–94.
- Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006;10:1319–29.
- Miller AR, Martenson JA, Nelson H, Schleck CD, Ilstrup DM, Gunderson LL, et al. The incidence and clinical consequences of treatment-related bowel injury. Int J Radiat Oncol Biol Phys 1999;43:817–25.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
- Schneider RA, Vitolo V, Albertini F, et al. High-dose, spot scanning based proton therapy for paraspinal/ retroperitoneal neoplasms and small bowel tolerance: Dose distribution analysis in a patient cohort. Proceedings of the 52nd Annual ASTRO Meeting. Int J Radiat Oncol Biol Phys 2010;78(Suppl):133 [Abstract].
- Glanzmann C. Late effects after high-voltage radiation therapy in the region of the minor pelvis: Experience with more than 1000 patients between 1950 and 1977. Strahlentherapie 1980;156:678–83.
- Letschert JG, Lebesque JV, de Boer RW, Hart AA, Bartelink H. Dose-volume correlation in radiation-related late small-bowel complications: A clinical study. Radiother Oncol 1990;18:307–20.
- Gallagher MJ, Brereton HD, Rostock RA, Zero JM, Zekoski DA, Poyss LF, et al. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. Int J Radiat Oncol Biol Phys 1986;12:1565–7.
- Wharton JT, Jones HW 3rd,Day TG Jr, Rutledge FN, Fletcher GH. Preirradiation celiotomy and extended field irradiation for invasive carcinoma of the cervix. Obstet Gynecol 1977;49:333–8.
- Piver MS, Barlow JJ. High dose irradiation to biopsy confirmed aortic node metastases from carcinoma of the uterine cervix. Cancer 1977;39:1243–6.
- Mak AC, Rich TA, Schultheiss TE, Kavanagh B, Ota DM, Romsdahl MM. Late complications of postoperative radiation therapy for cancer of the rectum and rectosigmoid. Int J Radiat Oncol Biol Phys 1994;28:597–603.
- Hoskin PJ, de Canha SM, Bownes P, Bryant L, Glynne Jones R. High dose rate afterloading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Radiother Oncol 2004;73:195–8.
- Wang CC, Schulz MD. The role of radiation therapy in the management of carcinoma of the sigmoid, rectosigmoid, and rectum. Radiology 1962;79:1–5.
- Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy?Acta Oncol 2010;49:378–81.
- Sun Myint A, Lee CD, Snee AJ, Perkins K, Jelley FE, Wong H. High dose rate brachytherapy as a boost after preoperative chemoradiotherapy for more advanced rectal tumours: The Clatterbridge experience. Clin Oncol (R Coll Radiol) 2007;19:711–9.
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, van Mastrigt GA, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev 2009;8:CD006269.