Abstract
Background. Limb salvage surgery (LSS) has gained widespread acceptance as the current treatment for treating extremity soft tissue sarcoma (STS) and has been greatly refined since its inception. Combined with improved adjuvant treatment modalities, rates of local relapse have greatly decreased. Nonetheless, local recurrence still occurs and identifying the cause and the subsequent effects of local recurrence can provide valuable insights as LSS continues to evolve. Methods. This retrospective study evaluated 278 patients treated for STS of the extremities between 2000 and 2006. Of these, 41 patients developed a local recurrence while 247 did not. Tumor characteristics and prognostic outcomes were analyzed. Wilcoxon rank sum test and either χ2 or Fisher’s exact was used to compare variables. Kaplan Meier and Gray's test for cumulative risk were also performed. Results. Patients who had a positive margin were 3.76 times more likely to develop local recurrence when compared to those with negative margins. This corresponds to a 38% risk of local recurrence if the margins were positive after six years vs. 12% if the margins were negative. In patients who underwent a re-excision, the presence or absence of residual disease upon re-excision did not have any bearing on local recurrence (p = 0.27). In comparing patients with and without local recurrence, there was no statistically significant difference in the rate and the proportion encountering distant metastasis and death due to sarcoma (p > 0.05). Conclusions. Despite advancements in surgery, radiation and imaging, positive margins still occur, and the presence of positive margins following definitive treatment continues to remain as a strong predictor for local recurrence. While local recurrence represents a negative outcome for a patient, its impact on future prognosis is influenced by a variety of factors such as time to local recurrence as well as the tumor's inherent biological characteristics.
Limb salvage surgery (LSS) has been the standard of care for extremity STS since the 1980s. As LSS continues to evolve, the risk of local recurrence has contrastingly decreased since its inception. However, the risk has not been eliminated completely, plateauing at the end of the 20th century. Various studies have been conducted since the inception of LSS and have shown factors such as positive surgical margins [Citation1–3], and high grade tumors [Citation4] as predictors of local recurrence. Since the approach to treating STS has changed considerably over time with regards to both continued refinement of LSS techniques and the improved adjuvant treatment and imaging modalities, we wanted to see if the factors that influenced local recurrence in extremity STS previously have changed since the turn of the century. Additionally, with the increasing numbers of STS that are inadvertently excised under the assumption of a benign tumor, we also wanted to see if the presence of residual disease upon re-excision affected prognosis.
While local recurrence represents a negative outcome for the patient, its impact on future prognosis remains a matter to be fully reconciled. To this end, our other goal was to compare prognostic outcomes, mainly distant metastasis and survival, in patients who developed a local recurrence and patients who did not develop a local recurrence at a major sarcoma center from 2000 to 2006.
Methods
We conducted a retrospective cohort study, following approval from the Institutional Review Board, at a major sarcoma center to examine the effects of various factors on local recurrence and subsequently the impact of local recurrence on overall prognosis since the turn of the century. All patients undergoing surgical resection of soft tissue sarcoma at our institution between January 2000 and December 2006 (n = 329) were identified based on a retrospective review of a prospective database and were considered for the study. Patients were excluded if they were younger than 18 years of age, if they lacked adequate medical records and if they had a tumor with good prognosis and borderline malignancy such as dermatofibrosarcoma protuberans [Citation5].
A total of 278 patients met the inclusion criteria and were initially subdivided into those who were treated with a single resection (n = 172) and those that had re-excision of a previously incompletely excised tumor (n = 106; all incompletely excisions were referred from outside institutions). This latter subset was further divided into two groups: those that had observable residual (either macroscopic or microscopic) disease (n = 29) and those that did not have any observable residual disease in the re-excised specimen (n = 77). In order to evaluate the impact of local recurrence on future prognosis, the 278 patients who met the initial inclusion criteria were subdivided into those who developed local recurrence (n = 41) and those that did not (n = 237).
Adjuvant radiation and chemotherapy were administered to the patient at the discretion of the multidisciplinary oncology team consistent with current standards of care. The median radiation dose used was 55.5 Gy. Chemotherapy, when used, consisted of postoperative anthracycline based regimens. It was recommended in patients with synovial sarcoma and in patients less than 40 years of age with high grade, deep tumors greater than 10 cm in size.
Patient demographics and tumor characteristics were collected from a retrospective review of medical records. Patient characteristics included age at time of surgery, sex, and race. Tumor characteristics consisted of size, depth (superficial or deep to the fascia of the underlying muscle), site (upper or lower extremity), histology grade (low, intermediate, or high), histologic subtype. Staging of the patients was also carried out per the guidelines recommended by the American Joint Committee on Cancer (AJCC) [Citation6]. Margins were either recorded as positive or negative for each patient. A positive margin was defined as the presence of malignant cells at the edge of the tissue where the ink is. The prognostic outcomes of death, distant metastasis, and local relapse due to STS were also abstracted from medical records. The time period from the index operation to each outcome listed above was measured in all patients.
Patient demographics, tumor characteristics and prognostic outcomes were compared across groups using Wilcoxon rank sum tests for continuous variables and χ2 or Fisher's exact tests. Survival curves for disease free survival, and metastasis free survival were calculated and presented using the Kaplan-Meier method [Citation7]. For the disease free survival curve, the primary end point of the study was designated as death due to sarcoma. Death was treated as a censored observation for patients who died from a cause not directly related to their STS. Gray’s test was calculated and used to compare the disease-specific hazards of death, and distant metastasis between the groups (local recurrence vs. no local recurrence) [Citation8,Citation9]. In addition to univariate comparisons, multivariable regression analyses were used to take into account potential confounders [Citation10]. This hazard ratio model examined positive/negative margins as the main independent local recurrence after controlling for primary/re-excision, tumor site (upper extremity vs. lower extremity), tumor depth, tumor grade, and tumor size.
Statistical software R (version 1.11.1, www.r-project.org) was used for all data analysis. Reported p-values were two-sided and a p-value of less than 0.05 was considered to indicate statistical significance.
Results
Positive margins predict local recurrence in both complete and incomplete resections
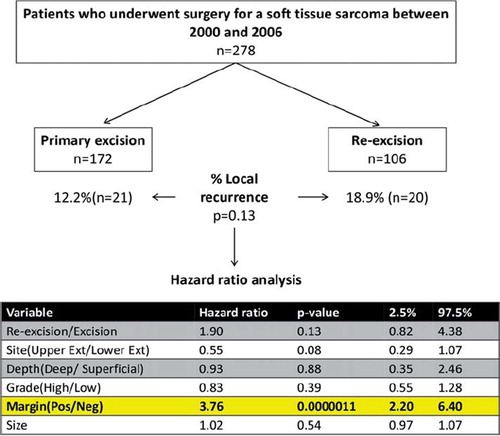
As depicted in , analysis of the 278 patients who were treated for soft tissue sarcoma of the extremity at our center revealed that 172 patients underwent a primary excision and the remaining 106 underwent a re-excision of their sarcoma following an incomplete initial resection. Comparison of the two groups showed that 12.2% of the patients from the primary excision group and 18.9% of the patients from the re-excision group developed local recurrence. However, this difference was not statistically significant (p = 0.13). Hazard analysis for sarcoma specific local relapse revealed that the presence of a positive margin was the strongest predictor of developing a local recurrence (p < 0.0001). The hazard ratio of developing local recurrence from a tumor that had positive margin was 3.76 (95% CI 2.20, 6.40) times that of a tumor that had negative margins following resection. Site, depth, size, grade and number of excisions were not found to be significant predictors of local recurrence.
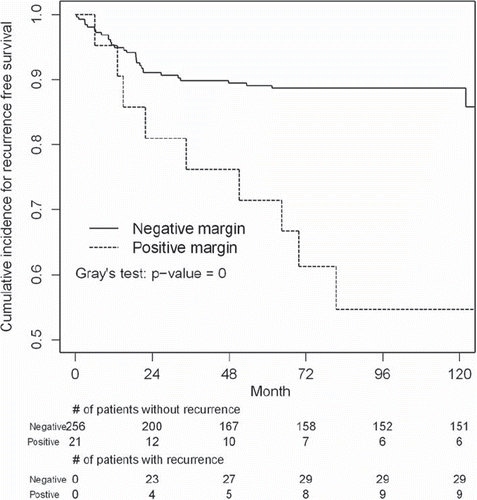
When time is factored in, the results indicate that the time to local recurrence for those with a positive margin is drastically different from those with a negative margin (Gray's test, p < 0.01). The cumulative incidence of recurrence free survival for patients with negative margins was much higher than for patients with positive margins. As shown in , at the end of 72 months, the estimated cumulative incidence of recurrence free survival due to sarcoma was 88% for patients with negative margins and 62% for patients with positive margins.
Presence of residual disease on re-excision does not predict local recurrence
As depicted in , the 106 patients with re-excised STS were divided into groups: those with residual disease (n = 77) and those without residual disease (n = 29). The mean follow-up time for the 106 patients was 4.4 years. Tumor location and other characteristics were not statistically different between the two groups (p > 0.05). On average, 24% and 22% of the tumors were superficial in the residual group and no residual group, respectively. Tumor grade was similar between the two groups with 58% of the tumors from both groups being high grade. When divided by the AJCC staging system, tumors were predominantly either Stage 1 or 2 (comprising a combined total of 63% of the tumors). Malignant fibrous histiocytoma (MFH) was the most common type of tumor seen in both groups, with 38% of the tumors being MFH in the residual group and 31% in the no residual group. The second most common type, however, was synovial sarcoma (14%) for the residual group and leiomyosarcoma (21%) for the no residual group.
Table I. Comparative demographics and tumor characteristics between residual and no residual group.
Prognosis between the residual group and the group without residual disease was measured by local recurrence, distant metastasis and death due to sarcoma. In the case of death due to sarcoma, it was found that 21% of the patients died due to the sarcoma in the residual group and 10% of the patients died due to the sarcoma in the no residual group. Although there was an increased proportion of patients experiencing death due to sarcoma in the residual group, the difference fails to be statistically significant (p = 0.39). In the case of local recurrence, the residual group had a 22% local recurrence rate while the no residual group had a 10% local recurrence rate. However, this difference again proved to be statistically insignificant (p = 0.27). In the case of distant metastasis, 29% of the patients in the residual group and 21% of the patients in the no residual group developed metastasis. This difference was also statistically insignificant (p = 0.41).
Local recurrence does not predict survival
Outcome measures studied for the impact of local recurrence on prognosis were distant metastasis and death due to sarcoma. Of the 278 patients who met the initial inclusion criteria as outlined in the methods section, 41 developed local recurrence and 237 patients did not. The median follow up time was 3.1 years for all 278 patients.
In comparing the two groups of patients as depicted in , statistically significant differences were seen in the margins obtained after surgery and the location of the tumor. Patients with a local recurrence were found to have a significantly higher proportion of positive margins following their surgery (p < 0.001). In addition, patients with local recurrences were also found to have a roughly equal proportion in the location of their tumors between the lower and upper extremity; this is significantly different from the 75% (lower extremity), 25% (upper extremity) split found in patients without local recurrences (p = 0.005).
Table II. Comparative demographics and tumor characteristics between recurrence and no recurrence group.
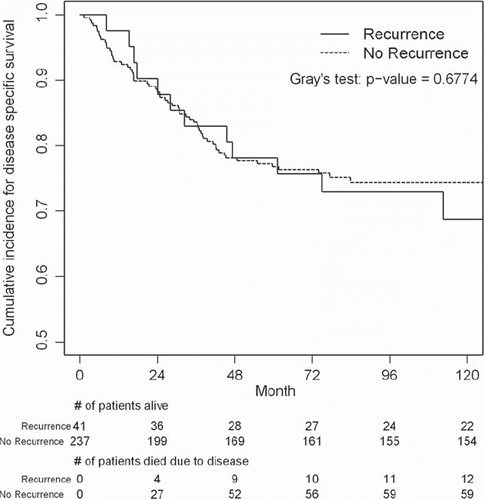
Sarcoma specific death. Of the patients experiencing local recurrence, a total of 13 patients (31.7% of the group) died as a consequence of their sarcoma. In the group without a local recurrence, a total of 59 patients (24.9% of the group) died as a consequence of their sarcoma. This difference was not statistically significant. In addition, when time was factored in, the results indicate that the time to death due to sarcoma was no different between the two groups (Gray’s test, p = 0.67). Death due to sarcoma per unit time was roughly equivalent in both the groups. The estimated cumulative incidence of death due to sarcoma, at the end of 100 months, was 27.1% for patients with a local recurrence and 25.7% for those without a local recurrence as indicated in .
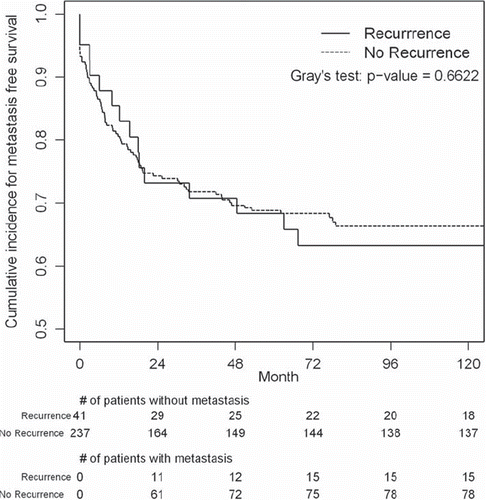
Distant metastasis. In the local recurrence group, 16 of the patients (39.0%) developed distant metastasis. In the group with no local recurrence, 78 of the patients (32.9%) developed distant metastasis. This difference was not statistically significant with a p-value of 0.44. Furthermore, the time to metastasis for the two groups was not statistically significant (Gray's test, p = 0.66). Patients who developed a local recurrence were not found to develop metastasis at a faster rate than patients without a prior local recurrence. By the end of 100 months from the time of surgery, the estimated cumulative incidence of metastasis was 36.8% for patients with local recurrence and 33.7% for those without as indicated in .
Discussion
LSS has established itself as the standard of care for extremity STS since the 1980s. However, the same controversies that surrounded its adoption have continued to plague it even as it continues to evolve – mainly local recurrence and its effect on overall survival. Various studies conducted immediately after the adoption of LSS have associated a variety of factors including high grade tumors [Citation4] and positive surgical margins [Citation1,Citation3,Citation11,Citation12] with the development of local recurrence. However, LSS has continued to evolve over the years with better refinement of technique. Simultaneously, adjuvant treatment and imaging modalities have also greatly improved.
Higher resolution imaging has allowed a more conservative resection and has enabled greater benefits to the patients in preserving their limb while still eradicating the tumor. In addition, adjuvant treatment modalities such as the administration of sterotactic radiotherapy have allowed for targeted therapeutics. Chemotherapy has also played an increasing role in the treatment of STS. The majority of chemotherapy regimens consist of anthracyline (i.e. Doxorubicin) based treatments that are usually administered postoperatively. Chemotherapy, in our patient population, was increasingly used in patients who developed metastasis (34% of patients with metastatic disease received chemotherapy) versus those who did not develop metastasis (11% of patients without metastatic disease received chemotherapy). Increasingly new means of implementing chemotherapy are also being tried. For example, regional hyperthermia at the tumor site has been shown to increase the benefits of existing chemotherapy modailites especially for high risk STS [Citation13].
Despite these improvements, positive margins still continue to remain as a strong positive predictor of local recurrence. This remained true even when other competeing factors such as grade, depth, size, etc of the neoplasm were taken into account. We found that there was no statistically significant difference in the proprotion of patients developing local recurrence between complete and incompletely excised sarcomas. In patients undergoing re-excision, the discovery of residual disease upon re-excision is frequently seen in many of our patients. Conversely, there are also patients who present for re-excision where no residual malignancy is found in the re-excised specimen. A comparison of our patients with and without residual disease demonstrated no significant difference in local recurrence, metastasis and death due to sarcoma. χ2-tests from our patient population revealed that there was no significant difference in the number of patients undergoing preoperative radiation therapy between the two groups. Thus, adjuvant radiation therapy prior to the re-excision is unable to explain the lack of residual disease in some of the re-excised specimens.
Residual disease has been implicated with increased mortality [Citation14,Citation15], increased risk of local recurrence [Citation14,Citation16,Citation17] and increased metastasis [Citation18,Citation19]. The standard of practice has thus been to pursue a more aggressive treatment regimen if residual disease is found upon re-excision. Our analysis reveals no statistical difference in local relapse, metastasis or death due to sarcoma between the two groups. However, it is possible that residual disease is often underreported due to the inability of the pathologist to view all of the cells in the specimen, especially with large re-excisions. In order to effectively treat tumors after undergoing incomplete resection, we believe re-excised tissue without residual disease should undergo the same treatment regimen as those found to have residual disease. Thus, all tumors with a previous incomplete excision with positive margin should be re-excised with adequate margins, ensuring appropriate adjuvant therapy regardless of the presence of residual disease on re-excision. This improves likelihood of adequate local control and in theory decreases local relapse [Citation20].
Local recurrence as a prognostic indicator
Local recurrence represents a negative outcome for patients following resection of a STS. However, varying results have been published on the effect of local recurrence on overall prognosis. Some authors have reported that local recurrence does not have a direct impact on distant metastasis [Citation21] or survival [Citation22–24]. Others claim that local recurrence portends a worse prognosis [Citation25,Citation26]. Specifically, local recurrences of intermediate to high grade have been associated with decreased survival [Citation27] and increased metastasis [Citation26]. Results from our sarcoma center over a six-year period revealed that patients with local recurrences experience distant metastasis and death at a rate that is no sooner than those without local recurrences.
In order to understand the varied results obtained by different groups in measuring the prognostic effects of local recurrence, it might be prudent to look at the explanations that initially brought about the local recurrence. These explanations have chiefly focused on inadequate initial resection as well as the tumor's inherent qualities. The latter is an important aspect of managing STS that should not be easily dismissed. The biological characteristics of the recurrent tumor help predict the way a tumor will behave and thus are important to consider when determining overall prognosis [Citation28]. More aggressive, higher grade tumors have a worse prognosis lower grade tumors and are more likely to result in distant metastases and death [Citation29]. Patients who undergo excision of a high grade tumor and subsequently develop a local relapse must have increased surveillance for systemic disease and systemic adjuvant therapy must be considered. Thus, not all recurrences of STS are the same, and it is possible for a tumor to recur with a higher grade.
In addition to tumor grade, time to local recurrence from the index operation may give additional information about the aggressiveness of a tumor [Citation30]. Assuming, negative margins were obtained following the initial resection, time to recurrence may provide information that takes into account both the tumor characteristics and the host environment. Tumors that have recurred quickly after surgery tend to be more aggressive in nature and could lead to increased metastasis and high mortality [Citation31]. Thus, similar to the high grade tumors that have recurred, these patients must have increased systemic disease surveillance and systemic adjuvant therapy must be considered. Further studies with larger number of patients can provide valuable information regarding the threshold for the time duration to local recurrence which puts the patient at an increased risk for subsequent distant metastasis and mortality.
The same tumor qualities that predispose some of the patients to local recurrence could also make them prone to subsequent poor prognosis. Thus it is also useful to consider patients who develop local recurrence but no distant metastasis as done by Gustafson et al. [Citation32]. This suggests that in this group of patients, the tumors that lack the ability to metastasize may have a better long-term prognosis than those that have demonstrated the ability to affect distant sites. As expected, and as reported by Gutafson et al., these patients with local recurrence but no distant metastases, signifying less aggressive tumors, have a much lower mortality due to their sarcoma [Citation32]. Thus, not all local recurrences are the same and one must ensure to not propose a blanket causative association of local recurrence with subsequent prognosis [Citation29].
Regardless of the effects of local recurrence on future prognosis, it represents increased costs and morbidity from having to undergo an additional surgical procedure for the patient [Citation33]. In addition, local recurrences also present significant emotional toils for the patient [Citation34]. As positive margins have been associated with local relapse in numerous studies, the best way to reduce local recurrence would be to excise tumors with proper margins. This is best done at specialized sarcoma centers [Citation28,Citation33,Citation34] where proper preoperative staging and planning is conducted prior to the excision of the STS.
Study limitations
Since this study looks at the various effects of STS resections post the turn of the century at a single institution, it is inherently underpowered in some of the analyses conducted. Thus, a beta error cannot be ruled out with certainty. However, the clinical relationships and conclusions postulated remain highly plausible.
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- Wilson RB, Crowe PJ, Fisher R, Hook C, Donnellan MJ. Extremity soft tissue sarcoma: Factors predictive of local recurrence and survival. Aust N Z J Surg 1999;69:344–9.
- Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg 2000;231:655–63.
- Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–15.
- Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, et al. High-grade extremity soft tissue sarcomas: Factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg 2003;237:218–26.
- Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, et al. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000;88:2711–20.
- Edge SB. AJCC cancer staging manual, 7th ed. New York, London: Springer; 2009.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 1999;18: 695–706.
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16: 1141–54.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509.
- Gerrand CH, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Bell RS, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br 2001;83: 1149–55.
- Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002;235:424–34.
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010;11:561–70.
- Rehders A, Stoecklein NH, Poremba C, Alexander A, Knoefel WT, Peiper M. Reexcision of soft tissue sarcoma: Sufficient local control but increased rate of metastasis. World J Surg 2009;33:2599–605.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008;90:203–8.
- Davis AM, Kandel RA, Wunder JS, Unger R, Meer J, O’Sullivan B, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997;66:81–7.
- Potter BK, Adams SC, Pitcher JD, Jr., Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008;466: 3093–100.
- Peiper M, Knoefel WT, Izbicki JR. [The influence of residual tumor on local recurrence after unplanned resection of soft tissue sarcoma]. Dtsch Med Wochenschr 2004;129:183–7.
- Fiore M, Casali PG, Miceli R, Mariani L, Bertulli R, Lozza L, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol 2006;13:110–7.
- Arai E, Nishida Y, Tsukushi S, Wasa J, Ishiguro N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res 2010;468: 3028–34.
- Gustafson P, Rooser B, Rydholm A. Is local recurrence of minor importance for metastases in soft tissue sarcoma? Cancer 1991;67:2083–6.
- Evans RA. Soft tissue sarcoma: The enigma of local recurrence. J Surg Oncol 1993;53:88–91.
- Brennan MF. The enigma of local recurrence. The Society of Surgical Oncology. Ann Surg Oncol 1997;4:1–12.
- Ueda T, Yoshikawa H, Mori S, Araki N, Myoui A, Kuratsu S, et al. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg Br 1997;79:553–7.
- Sabolch A, Feng M, Griffith K, Rzasa C, Gadzala L, Feng F, et al. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am J Clin Oncol 2012;35:151–7.
- Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 1997;15: 646–52.
- Novais EN, Demiralp B, Alderete J, Larson MC, Rose PS, Sim FH. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res 2010;468:3003–11.
- Moureau-Zabotto L, Thomas L, Bui BN, Chevreau C, Stockle E, Martel P, et al. Management of soft tissue sarcomas (STS) in first isolated local recurrence: A retrospective study of 83 cases. Radiother Oncol 2004;73:313–9.
- Brennan MF. Local recurrence in soft tissue sarcoma: More about the tumor, less about the surgeon. Ann Surg Oncol 2007;14:1528–9.
- Ramanathan RC, A’Hern R, Fisher C, Thomas JM. Prognostic index for extremity soft tissue sarcomas with isolated local recurrence. Ann Surg Oncol 2001;8:278–89.
- Eilber FC, Brennan MF, Riedel E, Alektiar KM, Antonescu CR, Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol 2005;12:228–36.
- Gustafson P, Dreinhofer KE, Rydholm A. Metastasis-free survival after local recurrence of soft-tissue sarcoma. J Bone Joint Surg Br 1993;75:658–60.
- Trovik CS, Gustafson P, Bauer HC, Saeter G, Klepp R, Berlin O, et al. Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand 2000;71:488–95.
- Trovik CS. Local recurrence of soft tissue sarcoma. A Scandinavian Sarcoma Group Project. Acta Orthop Scand Suppl 2001;72:1–31.