Case report
A 31-year-old man was diagnosed with a Breslow 1.4 mm depth malignant melanoma from the right side of his neck. Subsequent wide local excision of the scar showed no evidence of residual melanoma. Six years later he developed palpable left jugular chain lymph nodes confirmed by ultrasound guided needle aspiration as metastatic melanoma. A positron emission tomography (PET)-scan showed metabolically active lymph nodes in the inferior left neck and left supraclavicular fossa. Surgical resection revealed that two of 52 nodes were positive for metastatic melanoma. The patient emigrated to Australia and one year later he developed brain metastases and had this resected followed by whole brain irradiation. He also underwent a right nephrectomy for renal-confined melanoma metastases. He returned to Ireland 16 months later and a baseline computed tomography (CT)-scan suggested left supraclavicular recurrence. A PET-scan confirmed metabolically active disease in this area, extensive sub-diaphragmatic nodal disease as well as intramuscular disease in the left chest wall.
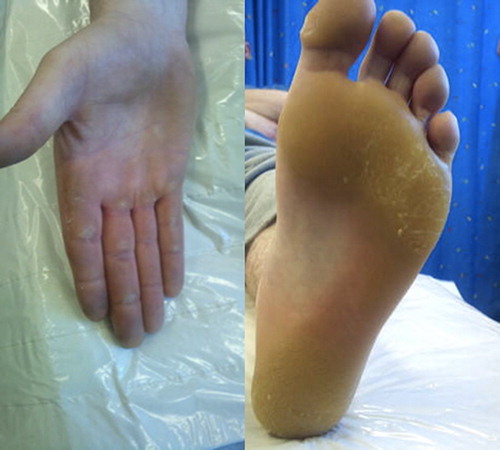
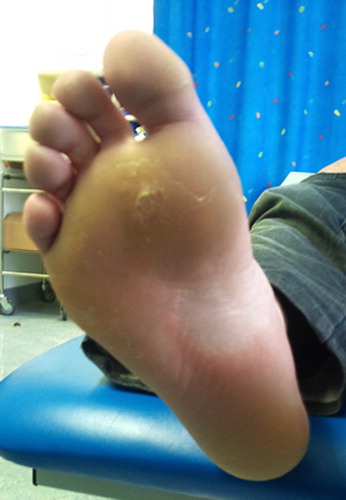
Mutational analysis using allele specific PCR was performed on archived tissue and confirmed the presence of the BRAF V600E activating mutation. The patient was enrolled in a clinical trial comparing an investigational oral BRAF inhibitor (dabrafenib) with standard dacarbazine chemotherapy. He was randomized to the investigational compound and commenced treatment in July 2011. At six-week follow-up he was noted to have painful grade II palmar-plantar hyperkeratosis impacting on his activities of daily living including walking and driving (). Interval CT-scan revealed a partial response based on RECIST (Response Evaluation Criteria In Solid Tumors). Following dermatology review, he was commenced on clobetasol (corticosteroid) 60% in polyethylene glycol 40% applied twice a day along with simple oral analgesia. Despite this the hyperkeratosis progressed to a grade III reaction with significant detrimental impact on his quality of life. The investigational drug was discontinued for a two-week period and recommenced as per protocol with a dose reduction. There was no discernable improvement in the hyperkeratosis and dermatology advised commencing acitretin 20 mg daily. At his most recent review after 50 weeks on study (dose reduction after 10 weeks) the skin toxicity had reduced to grade I with almost complete resolution of the hyperkeratosis and no new lesions evident (). Our patient continues on acitretin and the BRAF inhibitor compound.
Discussion
Approximately 40–60% of melanomas carry an activating mutation in the kinase domain of the BRAF gene [Citation1]. BRAF is a member of the RAF kinase family involved in the RAS/RAF/MEK/ERK kinase pathway. Activating mutations in BRAF are associated with increased growth and proliferation of cancer cells. Ninety percent of BRAF mutations result in a substitution of glutamic acid for valine at amino acid 600 (the V600E mutation).
The pivotal phase III trial (BRIM 3), comparing vemurafenib, an oral BRAF kinase inhibitor, to dacarbazine (DTIC) in treatment naïve patients with advanced metastatic melanoma, observed a significant impact on progression free survival and overall survival. At six months, overall survival was 84% in the vemurafenib group and 64% in the DTIC group; however, longer follow-up is required in order to establish the true impact on overall survival [Citation1]. More recently a randomized trial (BREAK 3), compared darafenib, an oral BRAF kinase inhibitor, with dacarbazine and reported a significant median progression free survival benefit of 2.4 months after a median of duration of follow- up of five months [Citation2].
To date the most frequent significant side effects reported with BRAF inhibitors are cutaneous, and include squamous cell carcinomas and keratoacanthomas, keratoderma/hyperkeratosis and desquamation [Citation2]. In phase I and II trials, 24% of patients treated with vemurafenib developed cutaneous lesions. This was slightly lower in the phase III trial with 18% of patients developing at least one cutaneous squamous cell carcinoma or keratoacanthoma [Citation1]. Hyperkeratosis occurred in 24–28%. Similarly, in the recently reported phase III trial comparing dabrafenib with dacarbazine (BREAK 3), the most common cutaneous adverse events in the dabrafenib arm included hyperkeratosis (37%) and palmar-plantar erythrodysesthesia syndrome (20%). Interestingly the incidence of squamous cell carcinoma/keratoacanthoma was lower in the dabrafenib trial (5%). Phototoxicity also occurred less frequently in patients than was seen in the vemurafenib trial (3% vs. 41%, respectively) [Citation2]. Skin changes often occur within the first months of therapy. The mechanism of development of cutaneous lesions is not fully understood. Studies have demonstrated that in non-cancerous cells lacking the V600E mutation, the inhibitor paradoxically activates downstream signaling of the mitogen activated protein kinase (MAPK) pathway, promoting growth [Citation3]. This, in turn, may explain why the combination of RAF and MEK inhibitors, used primarily to reduce resistance which develops with monotherapy, is associated with less skin toxicity and no epithelial skin tumors as has been reported in single agent therapy [Citation4].
Similar hand-foot reactions complicated by hyperkeratosis have been seen with sorafenib, a non-specific RAF kinase inhibitor with a spectrum of activity on a number of targets including the RAF-1 pathway, with hyperkeratosis reported in 30–60% of patients. This is also seen to a lesser extent with other multi-kinase inhibitors, e.g. sunitinib [Citation5,Citation6]. The reaction induced by RAF inhibitors exhibits several of the features of classic hand-foot syndrome, including erythema, paresthesias, fissuring, and non-specific inflammatory infiltrates. However, hyperkeratosis (palmar and/or plantar), typically yellowish, painful diffuse hyperkeratotic plaques located on the pressure areas, is not a common feature in classic hand-foot syndrome and is thought to be a unique effect of multi-tyrosine kinase inhibitors [Citation5]. Cutaneous lesions associated with multi-kinase inhibitors have been described as a continuum of abnormal keratinocyte proliferation, ranging from benign follicular cystic lesions to malignant squamous cell carcinoma [Citation3]. Histologic examination has found epidermal parakeratosis and dyskeratosis with band-like areas of necrotic keratinocytes and a dense perivascular lymphocytic dermal infiltrate [Citation6]. Dose reduction can decrease the symptoms but the hyperkeratosis usually persists [Citation7].
Recommended treatment for hyperkeratosis includes topical agents that inhibit keratinocyte proliferation [Citation8]. There are little data on topical or systemic therapy for BRAF induced hyperkeratosis. Acitretin is an oral second generation retinoid most commonly used in the treatment of resistant psoriasis. It is thought to work by slowing down the proliferation of the skin cells. It binds to nuclear receptors that regulate gene transcription inducing keratinocyte differentiation and reducing epidermal hyperplasia. Retinoids also down-regulate proto- oncogenes through activation of the retinoic acid receptor. There is no known potential interaction with RAF inhibitors. A recent case report has suggested that the use of acitretin may be effective in reducing the number of new malignant and benign skin lesions in patients being treated with BRAF inhibitors [Citation9].
Given the frequency and potentially debilitating nature of this problem, we propose that acitretin is a treatment option that can be administered safely with BRAF inhibitors in advanced melanoma.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16.
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358–65.
- Arnault JP, Mateus C, Escudier B, Tomasic G, Wechsler J, Holville E, et al. Skin tumours induced by sorafenib; paradoxical RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53 and TGFBR1. Clin Can Res 2012;18:263–72.
- Manousaridis I, Mavridou S, Goerdt S, Leverkus M, Utikal J. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol Epub 2012 Apr 28.
- Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008;19: 1955.
- Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, et al. Hand-foot skin reaction in patients treated with sorafenib: A clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008;158:592–6.
- Lountzis NI, Maroon MS. Sorafenib-induced palmoplantar hyperkeratosis. J Drugs Dermatol 2008;7:588–9.
- Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME, et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 2009;14:291–302.
- Anforth R, Cristina T, Blumetti MP, Affandi AM, Fernandez-Penas P. Systemic retinoid therapy for chemoprevention of non-melanoma skin cancer in a patient treated with vemurafenib. J Clin Oncol 2012;30:e165–7.