Abstract
Background. As previously demonstrated, tumor associated macrophages (TAMs) infiltration is associated with some cancers invasion and metastasis. However, the role of TAMs in the gastric cancer remains unclear. Methods Three- dimensional dynamic migration imaging system and real time RT-PCR were used to quantitatively investigate the effect of macrophages on the cancer cell mobility and gene expression related to cancer invasion and metastasis, including ADAM8, ADAM9, MMP9, TIMP3, VEGF-A and IL8 genes, in AGS, HGC-27, Hs-746T and NCI-N87 gastric cancer cell lines under normal or hypoxic conditions. Results. Under normal conditions, the cancer cell invasion rate was increased significantly and all six gene expressions were upregulated in all four cancer cell lines by macrophages. Under hypoxia the changes in the cancer cell invasion rate induced by macrophages was negatively correlated to the TIMP3 expression. In non- metastatic cell line AGS, the increase in migration rate induced by macrophages was further elevated under hypoxia with increased ADAM8 and ADAM9 expression and decreased MMP9 and TIMP3 expressions. Under hypoxia, the induction by macrophages for IL-8 expression was increased significantly in distant metastatic cell lines NCI-N87 and HS-746T, VEGF-A was increased in HGC-27 cell line. Conclusions. Both macrophages and hypoxia play an indispensable role in regulating the invasion of gastric cancer cells in vitro; ADAMs, MMP9 and TIMP3 might be involved in TAM induced invasive power of gastric cancer cells.
Gastric cancer is the second most common fatal carcinoma in the world, leading to more than 736 000 deaths annually according to the World Cancer Report 2008, which is issued by International Agency for Research on Cancer (IARC) [Citation1]. Accumulated experimental and clinical research reveals that chronic inflammation is also related to cancer formation or progression [Citation2]. Of the inflammatory causes for cancer, special macrophages called tumor associated macrophages (TAMs), play a central role in the tumor onset and progression [Citation3]. TAMs have been found recruitment in tumor tissues and related to shorter survival time of patients [Citation4,Citation5]. Ishigami et al. [Citation6] found that TAMs infiltration in the gastric carcinoma tissue was positively correlated with depth of invasion, nodal status and clinical stage, but Ohno et al. [Citation7] found that infiltration of TAMs was related to better five-year disease free survival. Therefore, the role of TAMs in gastric cancer is not confirmed, the mechanism of TAMs regulating the invasion and metastasis also remains unclear.
We have used three-dimensional (3D) dynamic migration imaging analysis system to accurately calculate the migration rate of four gastric cancer cell lines (AGS, HGC-27, Hs-746T and NCI-N87) in a matrix resembling the epithelial basement membrane. We have also measured the change in the expression of genes related to invasion and angiogenesis (ADAM8, ADAM9, MMP9, TIMP3, VEGF-A and IL-8) in these cell lines by real time RT-PCR. The effect of both macrophages and hypoxia for the migration rate, and the gene expression was defined.
By now, there are no reports about the expression of these genes in the relationship between gastric cancer and chronic inflammation. ADAM8 and ADAM9 belong to ADAM family which is an MMP-related metalloproteinase family and has multiple functions including cell adhesion, migration and proteolysis [Citation8]. TIMP3 (Tissue inhibitor of metalloproteinase 3) is an inhibitor of metalloproteases (MMP) [Citation9]. TIMP3 also reduces ADAM gene expression in some members of ADAM family (ADAM10, ADAM12 and ADAM28), but not the expressions of ADAM 8 and ADAM9 [Citation10].
In our study, we found that co-culture with macrophages increased the invasion rate of every gastric cancer cell line studied. There was a correlation between ADAM8 expression and increase in invasion rate due to macrophages. The changes in macrophage induced invasion rate by hypoxia were correlated with TIMP3 expression. On the other hand, in non-metastatic cell line grown with macrophage, the expressions of ADAM8, ADAM9 were upregulated by hypoxia, MMP9 and TIMP3 expressions were downregulated resulting in highly increased invasion rate.
Materials and methods
Gastric cancer cells culture
Four gastric cancer cell lines with different biological behavior characteristics, AGS, HGC-27, Hs-746T and NCI-N87, were cultured in Ham's F12K medium, EMEM medium, Dulbecco's modified Eagle's medium (DMEM) and RPMI-1640 medium, respectively. The site of derivation of the cell line was stated in ().
Table I. Source of gastric cancer cell lines.
Isolation of monocytes and macrophages
Mononuclear cells were isolated from healthy subject's blood with density gradient centrifugation (Ficol-Paque, Amershamn, Uppsala, Sweden). The mononuclear cells layer was transferred to a clean tube and centrifuged. The cells were counted and 1.4 × 106 cells were placed on Matrigel (Matrigel, BD Biosciences, San Jose, CA, USA) on coverslip (Nalge Nunc International Corporation, Naperville, German). The isolated cells were grown in serum-free medium designed for macrophages (Macrophage serum free medium, Gibco, Paislay, UK) with granulocyte-macrophage colony stimulating factor (GM-CSF, 10 ng/ml, ImmunoTools, Oldenburg, Germany), antibiotics and 5% CO2 at 37°C. Monocytes adhered to the matrigel over night and differentiated to macrophages by GM-CSF in six days and then used for the experiment. When co-cultured with cancer cells, macrophages developed into TAMs [Citation11] with special surface marker, CD14+. After co-culturing with gastric cancer cells, the percentage of CD14+ positive macrophages reached 82% as measured with flow cytometry.
Invasion and migration assay (3D dynamic migration imaging system)
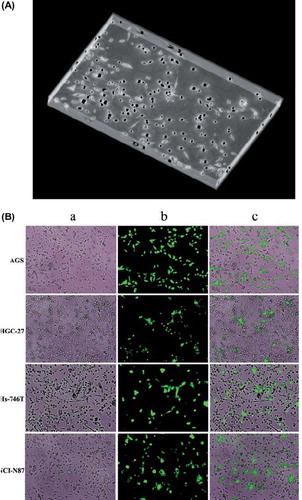
Cells were grown on matrigel covered coverslip wells with serum-free medium designed for macrophages. Gastric cancer cells were grown either alone or with differentiated macrophages on matrigel and either in normal (5% CO2 in air) or hypoxic conditions (5% CO2, 2% O2, N2 94%). Sixty thousand gastric cancer cells were seeded in each well of the coverslip. Gastric cancer cells were stained with fluorescent dye (CellTracker green CMFDA, Invitrogen, Eugene, OR, USA) before imaging. During the invasion phase the cancer cells invaded in matrigel were imaged by 3D dynamic migration imaging system (Olympus A × 70 Research System microscope, Japan; 12Bit Cooled Imaging Sensicam camera, PCD Imaging, Kelheim, Germany). The average migration speed was calculated from the cells which could be tracked at least for 6 h in one z-plane (ImagePro Plus, Media Cybernetics, Bethesda, MD, USA) ().
Figure 1. Gastric cancer cells were grown with macrophages in Matrigel. (A) 3D picture screened by 3D dynamic migration imaging system. (B) AGS, HGC-27, Hs-746T and NCI-N87 gastric cancer cell cultured with macrophages. (a) image of gastric cancer cells with macrophages under normal light, ×100 magnification; (b) image of gastric cancer cells stained with fluorescence, ×100 magnification; (c) combined image, ×100 magnification.(N = 3).
Cell sorting by magnetic separation
Cell sorting was performed after gastric cancer cells were cultured with macrophages for 24 h. Cell sorting was processed by MACS separator (MACS Miltenyi Biotec, Germany), which is based on magnetic separation. After Matrigel contained cells were dissolved and centrifuged, 80 μl of Buffer (degassed PBS with 0.5% BSA and 2 mM EDTA) and 20 μl CD14 MicroBeads (MACS Miltenyi Biotec, Germany) were added to the deposit and incubated for 20 min at 4°C. Then the cells were washed by Buffer and centrifuged. Cells resuspended with Buffer were transferred to the prepared LS column (MACS Miltenyi Biotec, Germany). Total effluents which contained gastric cancer cells and CD14 negative macrophages were collected and centrifuged. The cell pellets were resuspended in Buffer and CD11b Micro Beads, and the above mentioned separating protocol was repeated.
Real time RT-PCR
Total-RNA was isolated from the sorted cells by membrane binding (Rneasy Mini Kit, 74104, Qiagen, Hilden, Germany). RNA was reverse transcribed to single stranded cDNA by reverse transcriptase method (High Capacity cDNA Reverse Transcription Kit, 4368814, Applied Biosystems, Bardburg, NJ, USA). The target gene expression was measured by real time RT-PCR method (TaqMan gene Expression Assay, Applied Biosystems). GAPDH was used as an endogenic control (TaqMan Endogenous Controls, Applied Biosystems). The PCR reactions were run in ABI PRISM 7000 sequence detection system. 2-ΔΔCT referred to the fold of the relative mRNA expression of one sample as compared to the calibration sample. 2-ΔCt was defined as the fold of relative mRNA expression of target gene as compared to GAPDH expression in the same sample.
Western blotting
The cells were lysed by adding lysing buffer (pH 7.6) containing 150 mM NaCl, 10 mM TRIS-HCl, 1 mM EDTA, 1 mM EGTA, 1% TritonX-100, 0.5% NP-40, 1x Complete™ protease inhibitor cocktail (Roche, Mannheim, Germany) and 1 mM Pefabloc SC (Roche, Mannheim, Germany) and by incubating for 1 h at 0°C followed by short vortexing. Protein concentrations of the samples were determined by colorimetric Bradford assay and equal amounts of cytoplasmic protein extracts (20 μg) were diluted in Laemmli sample buffer with 5% mercaptoethanol. After incubation for 5 min at 95°C, the samples were resolved in 10% polyacrylamide gels in Tris-glycine-SDS buffer. The gels were transferred to nitrocellulose membranes, and blocked in Odyssey blocking buffer (927-40000, LI-COR, Lincoln, NE, USA). The blots were then incubated overnight with rabbit anti-ADAM8 (AB19017, Millipore, Temecula, CA, USA) and monoclonal mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (sc-32233, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in Odyssey blocking buffer + Tween 0.1% (OBBT). The membranes were washed three times in PBS + 0.1% Tween (PBST) and incubated for 1 h at room temperature with goat anti-rabbit IgG IRDye 800 (1:15000 (v/v), 926-31220, LI-COR, Lincoln) and with goat anti-mouse IgG Alexa 680 dye (1:15000, A21058, Molecular Probes, Eugene, OR, USA) in OBBT. After washing, protein bands in the membranes were scanned by Odyssey infrared imager (LI-COR, Lincoln).
Statistical analysis
Statistical Package for Social Science (SPSS) version 17.0 was used. Data were expressed as means ± SEM. One way ANOVA was used to compare the cell movement speed data and mRNA expression data between different groups. P-values were considered significant at the p < 0.05 level.
Results
Effect of macrophages on the invasion rate of AGS, HGC-27, Hs-746T and NCI-N87 cells under normal or hypoxia conditions
Under normal conditions, the cell's invasion rates were significantly upregulated by macrophages in all cell lines (). In distant metastasis cell lines the macrophage induced increase in invasion rates were significantly larger than in non-metastatic cell line, AGS [1.3 ± 0.1, 1.7 ± 0.1 (p < 0.001) and 3.2 ± 0.1 (p < 0.001) with/without macrophages for AGS, NCI-N87 and Hs-746T cell lines, respectively].
Figure 2. Effect of macrophages on the mobility of gastric cancer cells under normal and hypoxic conditions. The cells movement rates were accelerated due to macrophages in AGS, HGC-27, Hs-746T and NCI-N87 cell lines under normal condition. Hypoxia induced increase in migration rate in AGS and HGC cell lines, decrease in Hs-746T and NCI-N87 cell lines without macrophages. When the cancer cells with macrophages cultured together under hypoxia condition, the cells movement speeds were increased in AGS, HGC-27 and NCI-N87 cell line compared with normal condition with macrophages, but decreased in Hs-746T cell line.*P < 0.05; **P < 0.001.(N = 3).
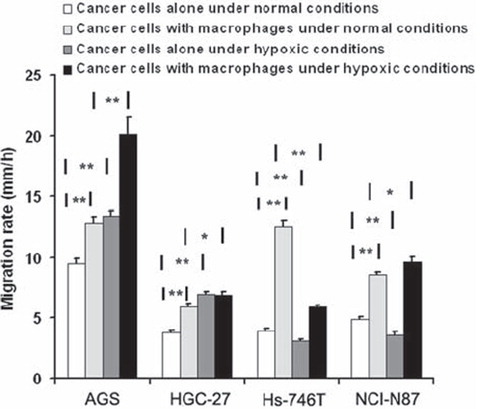
Under hypoxic conditions without macrophages, the invasion rates were accelerated significantly in AGS and HGC-27 cell lines, but were reduced in distant metastasis cell lines Hs-746T and NCI-N87.
When the cancer cells were cultured with macrophages under hypoxic conditions, the invasion rate increased significantly in AGS, HGC-27 and NCI-N87 cell lines compared to normal conditions with macrophages, but decreased significantly in Hs- 746T cell line (p < 0.001) (, Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718444).
Role of macrophages in the gene expressions related to invasion and angiogenesis under normal or hypoxic conditions in gastric cancer cells
ADAM8, ADAM9, MMP9, TIMP3, VEGF and IL-8 expression in gastric cancer cell lines. The expressions of ADAM8, ADAM9, MMP9, TIMP3, VEGF and IL-8 relative of GAPDH expressions are shown in . It is noteworthy that, TIMP3 expression was the highest in HGC-27 cell line, having the lowest cell invasion rate during normal conditions. Also, MMP9, VEGF-A and IL-8 expressions relative to GAPDH expression in distant metastasis cell lines were significantly higher than in non- metastatic AGS cell line ().
Table II. ADAM8, ADAM9, MMP9, TIMP3, VEGF-1 and IL-8 mRNA relative expression in gastric cancer cell lines.
Effect of macrophages on ADAM8, ADAM9, MMP9, TIMP3, VEGF and IL-8 expression in gastric cancer cell lines under normal conditions. All detected genes were upregulated (p < 0.05) in all four gastric cancer cell lines, when co-cultured with macrophages under normal conditions (, Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718444).
Figure 3. Effect of macrophages on ADAM8, ADAM9, MMP9, TIMP3, VEGF-A and IL-8 expression in gastric cancer cell lines under normal or hypoxic conditions, log(mRNA relative expression). Different gastric cancer cell lines were cultured with or without macrophages either in normal or hypoxic conditions. Expressions of the above mentioned genes were measured with realtime RT-PCR method. Under normal conditions, macrophage co-culture upregulated all six genes in gastric cancer cells. Hypoxia had effects depending on cell line, but in general hypoxia tended to decrease MMP9 expression and increase TIMP3 expression in gastric cancers cells grown with or without macrophages. *P < 0.05; **P < 0.001, as compared to AGS cell line. # the relative mRNA results is significant at P < 0.05 level.(N = 4).
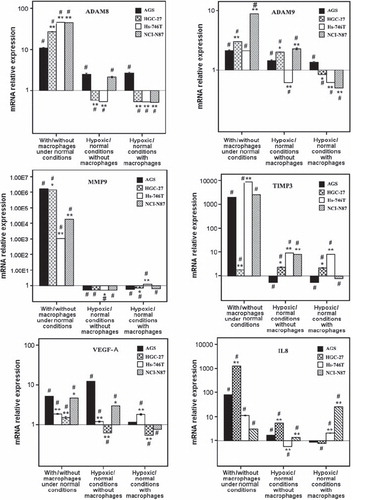
ADAM8 expression was elevated more than 10-fold in each cell line. The elevation of ADAM8 expression was higher in metastatic cell lines than in non-metastatic cell line and the ADAM8 expression correlated with macrophage induced increase in invasion rate ().
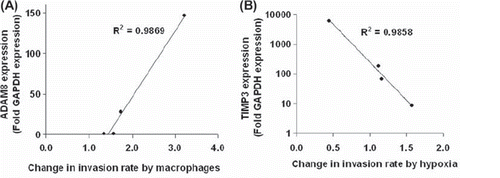
Figure 4. Correlations between ADAM8 and TIMP3 expressions in cancer cells and changes in invasion rate either by macrophages or by hypoxia. (A) ADAM8 expression in different cancer cell lines (dots) correlates with their response in invasion rate as the cancer cells are co-cultured with macrophages (p = 0.006, N = 4). (B) TIMP3 expression in different cancer cell lines (dots) correlates negatively with changes in invasion rate induced by hypoxia (p = 0.007, N = 4).
MMP9 expression was upregulated in cancer over 1 × 104-fold due to macrophages. The upregulations of MMP9 expression in metastatic cell lines were not as intense as in non-metastatic AGS cell line.
The upregulations of VEGF altered between the cell lines (, Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718444).
Effect of hypoxic conditions on ADAM8, ADAM9, MMP9, TIMP3, VEGF and IL-8 expression in gastric cancer cell lines without macrophages. ADAM8 expression was downregulated in HGC-27, Hs-746T cell lines, but upregulated in AGS and NCI-N87 cell line under hypoxic conditions. Hypoxic conditions induced an increase of ADAM9 expression in AGS, HGC-27 and NCI-N87 cell lines and a decrease in Hs-746T cell line.
MMP9 expression was downregulated in all four cell lines by hypoxia. TIMP3 expression was upregulated in all metastatic cell lines by hypoxia and downregulated in AGS cell line.
Both VEGF-A and IL8 were upregulated in AGS, HGC-27 and NCI-N87 cell lines, and decreased in Hs-746T cell lines under hypoxic conditions. The upregulation of VEGF-A was most prominent in AGS cell line, and the upregulation of IL-8 expression by hypoxia was most obvious in HGC-27 cell line. (, Supplementary Table III available online at http://informahealthcare.com/doi/abs/ 10.3109/0284186X.2012.718444).
Effect of hypoxia on ADAM8, ADAM9, MMP9, TIMP3, VEGF and IL-8 expression in gastric cancer cell lines cultured with macrophages. We found that the hypoxia induced ADAM8 and ADAM9 expressions elevations in the AGS cell line cultured with macrophages, as compared to normal conditions co-cultured with macrophages, but decreased in all metastatic cancer cell lines. It is interesting that also the corresponding invasion rate was increased in AGS cell line, but was only modestly increased or decreased in invasive cell lines.
MMP9 expression was upregulated slightly in Hs-746T and downregulated in all other cell lines by hypoxia. TIMP3 expression was elevated in the HGC-27 and Hs-746T cell lines, and decreased in AGS and NCI-N87 cell lines under hypoxia. There was a negative correlation between TIMP3 expression and change in invasion rate by hypoxia ().
VEGF-A expression was increased in AGS and HGC-27 cell line under hypoxia, but downregulated in Hs-746T and NCI-N87 cell lines during presence of macrophages. The IL-8 expressions were increased in distant metastasis cell lines by hypoxia and downregulated in AGS and HGC-27 cell lines during presence of macrophages. (, Supplementary Table IV available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718444).
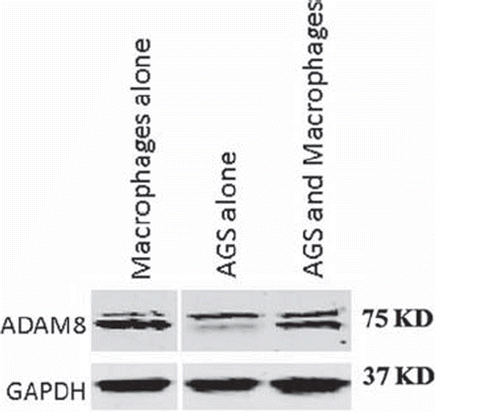
ADAM8 protein expression in AGS cell line. ADAM8 protein level expression was measured in macrophages grown alone, in AGS cells grown alone and in macrophage-AGS co-culture. The main band of ADAM8 in macrophage was detected slightly lower than in AGS, but no difference was observed when macrophages and ADAM were grown together (, n = 3) [Supplementary Tables I-IV available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718444].
Discussion
Clinical evidence shows that inflammation plays an important role in cancer initiation and progression. Solid tumors are infiltrated by macrophages, which comprised up to 80% of tumors [Citation12]. Macrophages appear in varying phenotypes based on environmental stimuli [Citation13], and are divided into two groups: M1 phenotype and M2 phenotype. TAMs are considered as an M2 phenotype, which has poor antigen presenting ability, produce factors that suppress T-cell proliferation and activity, and promote tumor growth and dissemination [Citation14]. Compelling evidence has emerged in recent years for TAMs playing an important role in tumor cell invasion, metastasis and survival [Citation15,Citation16]. The effect of the macrophages on the invasion and angiogenesis for gastric cancer still lacks confirmed evidence. Furthermore, the role of hypoxia in regulating macrophages function in gastric cancer is unknown.
In our study, the effect of macrophages on the movement of gastric cancer cells in Matrigel was detected. In normal conditions, the cell movement speed was accelerated significantly in all gastric cancer cell lines, when the cells were co-cultured with macrophages. The invasion rate of distant metastasis cell lines were increased significantly more by macrophages than in non-metastatic cell line. Moreover, it is interesting that the basic cell movement speed without macrophages was lower in all metastatic gastric cancer cell lines. However, since we have used only one primary cell line, the comparison between metastatic state is only suggestive and these results need to be confirmed.
It has been shown that TAMs preferentially localize to poorly vascularized regions of tumor [Citation17,Citation18], and the number of TAMs was higher in tumors containing high overall levels of hypoxia, as seen in primary human breast carcinomas and various animal tumors [Citation19,Citation20]. As previous studies have shown, hypoxia could induce the change of the phenotype of TAMs and promote proliferation, invasion, and metastasis of cancer cells [Citation21,Citation22]. In our study, under hypoxic conditions, whatever with or without macrophages, the cell migration rate was accelerated significantly in non-metastatic cell line, but retarded in aggressive distant metastasis cell lines, such as Hs-746T. Therefore, high aggressive gastric cancer cell lines might be negatively affected by hypoxia.
In fact, hypoxia could decrease the activity of TAMs in some points. Grimshaw and colleagues [Citation23] found that hypoxia decreased the activity of TAMs by upregulation of the mitogen-activated protein kinase phosphatase (MKP-1) in macrophages, which lead to rapid de-phosphorylation of the signaling enzymes (MEK, ERK1/2, and p38 MAPK) in the chemo-attractant receptor of TAMs. This progress terminated the chemotactic response from the chemokines secreted by tumor cells, and decrease the activity and mobility of TAMs [Citation24,Citation25].
TAMs promote cancer progression through several mechanisms, including promotion of invasion and migration and angiogenesis. In this study, we measured the expression of genes related to cancer invasion and metastasis in gastric cancer cell lines. We found that all six genes were upregulated in all gastric cancer cell lines by macrophages under normal conditions. Moreover, we found that the most extensive upregulation of ADAM8 expression by macrophages under normal conditions occurred in cell lines, which also showed most prominent increase in invasion rate induced by macrophages. The correlation between ADAM8 expression and increase in invasion rate induced by macrophages suggests a role for ADAM8 in invasion. Hypoxia downregulated these macrophage-induced ADAM8 and ADAM9 expressions in metastatic cancer cell lines, but not in non-metastatic cell line, since hypoxia mainly downregulated invasion rate in distant metastatic cell lines, these results might suggest that these ADAMs are involved in invasive power of these cells, although our results are preliminary.
MMP9 is confirmed to be related to gastric cancer invasion and migration [Citation26]. In this study, we found that MMP9 was strongly upregulated by macrophages in all four cell lines under normal conditions. However, hypoxia decreased the macrophage-induced upregulation of MMP9 expression in all other cell lines, but not in Hs-746T. Although the increase in MMP9 expression during macrophage co-culture correlated with increased migration rate, during hypoxia this correlation was not apparent. Factors other than MMP9 seem to affect the migration rate under these conditions.
TIMP3 as an inhibitor of MMP9 was upregulated by macrophages in all four cell lines under normal conditions. This might reflect a negative feedback mechanism of the large increase in MMP9 expression. However, under hypoxia, whatever with or without macrophages co-cultured, TIMP3 expression was upregulated most in Hs-746T cell lines, which also showed decreased migration rate due to hypoxia probably originating from the inhibitory effect on the proteolysis meditated by MMP9. Also the negative correlation between TIMP3 expression and hypoxia induced change in invasion rate in macrophage co-cultured cancer cells suggests that TIMP3 is one of the regulatory factors and hypoxia, although further studies are needed to confirm this observation.
Formation of new blood vessels (angiogenesis) is essential to supply oxygen and nutrients to tumor [Citation27]. It has been shown that angiogenesis is facilitated by TAMs via expression of angiogenic factors (such as vascular endothelial growth factor VEGF [Citation28] and IL-8 [Citation29]) promoting the spread of vascular endothelial cells. Tumor angiogenesis also could be promoted by hypoxia, resulting in enhanced expression of angiogenic factors [Citation30]. In our study, we found that VEGF-A and IL-8 expression were upregulated by macrophages in four gastric cancer cell lines under normal conditions. However, during macrophage co-culture, the regulation of VEGF- A and IL-8 by hypoxia depended on the cell line.
Gastric cancer patients have unfavorable therapeutic results and poor survival. Based on the result of pro-tumoral functions of TAMs, TAMs appeared as attractive candidate of novel therapeutic strategies. We found hypoxia could inhibit the effect of macrophages on mobility and expression of genes related to invasion and angiogenesis in highly aggressive cell lines, such as Hs-746T cell. On the contrary, it may promote that effect in the less aggressive gastric cell lines, such as the AGS cell line. Although our data is preliminary in vitro, the information on TAMs might someday provide new insights into improving the treatment of gastric cancer.
Supplementary Table IV
Download PDF (417.4 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from Sigrid Juselius Foundation and Helsinki University Central Hospital Research Funds.
References
- World Cancer Report 2008. Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.php [cited 2011 March 1]
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett 2008;267:204–15.
- Pollard JW. Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer 2004;4:71–8.
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009;9:259–70.
- Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: Relation to clinicopathological variables and survival. Int J Oncol 2000;17:445–51.
- Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res 2003;23:4079–83.
- Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 2003;23:5015–522.
- Turner SL, Blair-Zajdel ME, Bunning RA. ADAMs and ADAMTSs in cancer. Br J Biomed Sci 2009;66:117–28.
- Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 1998;58:2310–5.
- Ahonen M, Baker AH, Kahari VM. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett 2002;524:154–8.
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55.
- Bingle L, Brown NJ, Lewis CE. The role of tumor-associated macrophages in tumor progression: Implications for new anticancer therapies. J Pathol 2002;196:254–65.
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211–7.
- Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation. Blood 2006;107:2112–22.
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony- stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193:727–40.
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate- liposome-mediated depletion of tumor-associated macrophages: A new and highly effective antiangiogenic therapy approach. Br J Cancer 2006;95:272–81.
- Lewis CE, Pollard JW. Distinct role of macrophagesin different tumor microenvironments. Cancer Res 2006;66:605–12.
- Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, et al. Relation of hypoxia-inducible factor-2 α (HIF-2α) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res 2002;62:1326–9.
- Leek RD, Landers RJ, Harrism AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999;79:991–5.
- Collingridge DR, Hill SA, Chaplin DJ. Proportion of infiltrating IgG binding immune cells predict for tumor hypoxia. Br J Cancer 2001;84:626–30.
- Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 2003;198:1391–402.
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 2000;157:411–21.
- Grimshaw MJ, Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation— a potential mechanism. Eur J Immunol 2001;31:480–9.
- Ashida N, Arai H, Yamasaki M, Kita T. Differential signaling for MCP-1-dependent integrin activation and chemotaxis. Ann N Y Acad Sci 2001;947:387–9.
- Wain JH, Kirby JA, Ali S. Leucocyte chemotaxis: Examination of mitogen activated protein kinase and phosphoinositide 3-kinase activation by monocyte chemoattractant proteins- 1,-2,-3 and -4. Clin Exp Immunol 2002;127: 436–44.
- Sampieri CL, de la Peña S, Ochoa-Lara M, Zenteno-Cuevas R, León-Córdoba K. Expression of matrix metalloproteinases 2 and 9 in human gastric cancer and superficial gastritis. World J Gastroenterol 2010;16:1500–5.
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N Engl J Med 1971;285:1182–6.
- Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology 2010;215:242–9.
- Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, et al Upregulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res 2003;9:729–37.
- Chen J, De S, Brainard J, Byzova TV. Metastatic properties of prostate cancer cells are controlled by VEGF. Cell Commun Adhes 2004;11:1–11.