Abstract
Background. Patient-reported outcomes (PROs) and assessments of treatment-related toxicity provide important information on the effect of palliative chemotherapy and/or radiotherapy. The aim of this study was to review the effect of palliative radiotherapy and/or chemotherapy on symptoms and quality of life assessed by PROs and measurement of toxicity for patients with oesophageal cancer. Methods. The Central, Medline and Embase databases (1990 to November 2011) were systematically searched for prospective studies of palliative chemotherapy and/or radiotherapy in patients with advanced oesophageal cancer with PRO- and/or toxicity outcomes. The risks of bias were assessed. Results. Of 2677 records identified, only 32 included PROs, of which eight were randomised controlled trials. In studies with sufficient standard of PRO (n = 18), either Health Related Quality of Life (HRQL) (n = 14) or patient-reported dysphagia (n = 4), were assessed. Docetaxel added to cisplatin + fluorouracil (CF) improved HRQL compared to CF only, even though toxicity increased. Epirubicin added to CF resulted in longer preserved HRQL than its comparator in two trials, and non-inferiority in one. All phase II chemotherapy studies reported maintained HRQL or improved dysphagia combined with low level of toxicity. Brachytherapy resulted in better HRQL compared to stent placement in two trials, and external radiotherapy relieved dysphagia. The quality of the HRQL methodology and the interpretation and presentation of the PRO results varied, and clinical significance was seldom discussed. Conclusion. PRO endpoints are seldom used and further studies of homogenous patient groups with valid measures and methodology of PROs should be encouraged in the evaluation of palliative treatment. Brachytherapy, external radiotherapy and combination chemotherapy improved HRQL and dysphagia in the few identified studies with sufficient PRO methodology.
According to the literature, no studies of oesophageal cancer patients have shown a survival benefit of palliative chemotherapy or radiotherapy [Citation1,Citation2].Therefore, the treatment is given to prevent or palliate symptoms, and relevant endpoints must be considered when evaluating the effect of the palliative treatment.
Both the disease and the treatment have major impact on these patients’ symptoms, including dysphagia, functional well-being and health related quality of life (HRQL) [Citation3]. These concepts are best known by patients, and are therefore assessed using patient-reported outcome (PRO). During the last two decades guidelines to ensure sufficient quality of PRO methodology have been developed [Citation4,Citation5]. The use of PROs has increased significantly and they are now recognised as valuable endpoints in clinical trials [Citation6]. The traditional evaluations of survival, tumour response and time to progression, seem to be less appropriate. However, these endpoints are still often assessed in palliative studies.
Toxicity is another important issue when evaluating palliative treatment. Observer-rated toxicity of treatment is included in most clinical trials, e.g. National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) [Citation7]. Observer-rated patients’ performance status (ECOG/WHO or Karnofsky performance status) may also be regarded as a measure of toxicity of treatment.
The aim of this study was to systematically review the effect of palliative chemotherapy and/or radiotherapy for patients with advanced cancer of the oesophagus by assessing PROs, including HRQL, and toxicity after treatment. The primary outcome was the effect of palliative radiotherapy and chemotherapy assessed by PROs after treatment. The secondary outcome was treatment toxicity evaluated by health workers. In addition, the quality of the PRO methodology and to what extent the authors included results of the PROs in their conclusions, were evaluated.
Materials and methods
A protocol was written in accordance with the guidelines of the Cochrane handbook for systematic reviews [Citation5] in collaboration with The Norwegian Knowledge Centre for the Health Services.
Criteria for inclusion of studies
Prospective studies on patients with carcinoma of the oesophagus and oesophagogastric junction treated with chemotherapy and/or radiotherapy with palliative intent were included if PRO and/or toxicity were part of the treatment evaluation. Both randomised controlled trials (RCTs) and non-randomised trials were included to ensure sufficient data on the outcome measures. Studies of high-dose radiotherapy (≥ 40 Gy with concomitant chemotherapy or ≥ 50 Gy alone) were defined as treatment with curative intent if not stated otherwise, and were not included. Chemotherapy applied without surgery or radiotherapy was defined as treatment with palliative intent if not stated otherwise.
Questionnaires completed by the patients themselves or filled in by study personnel based on interviews of the patients (not observer-rated) were regarded as valid measurements. PROs regarding patient's satisfaction with care, mood disturbances and coping strategies, and toxicity not recognisable by the patients (e.g. haematological or nephrological toxicity), were not included.
Search methods for identification of studies
Searches were conducted in accordance with the guidelines of the Cochrane library [Citation5]. Two healthcare librarians with experience in search strategies performed the search in cooperation with the first author.
The Cochrane Central Register of Controlled Trials (Central), Medline and Embase databases (1990 to November 2011) were searched to identify relevant published trials. PRO was rarely used for evaluation of oesophageal cancer treatment before 1990 [Citation8]. A combination of subject headings and text words relating to chemotherapy and radiotherapy for oesophageal cancer with non-curative intent was used (Supplementary Appendix 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.731521). There were no restrictions related to language or study design. In addition, reference lists of identified PRO articles and reviews were manually searched for additional studies. Studies published in languages other than English, French, German, and the Scandinavian languages were identified, but not included due to lack of translation resources. Duplicates, abstracts and studies of less than 20 patients were excluded.
Data collection
All identified titles were reviewed by the first author (CDA), and titles definitely failing inclusion criteria were removed. Thereafter, titles and abstracts of articles were reviewed by pairs of three of the authors (CDA, ABJ, KB) using an eligibility assessment form. Discrepancies were resolved through discussion within the pairs and review by the third author if needed. Reasons for inclusion/non-inclusion were documented. If needed, the full text was reviewed before the final decision on inclusion was made. Data on the included studies were formally extracted by the first author and thereafter reviewed by one of the three others (ABJ, MGG or KB). The search details were presented according to the PRISMA 2009 flow diagram [Citation9].
Assessment of treatment effect, quality of PRO and risk of bias
For each study, the effect of treatment was described. The heterogeneity of the study population, treatment modalities and outcome measures did not allow a meta-analysis to be performed.
The PRO methodology must hold sufficient quality in order to be a valid treatment outcome. The current review was based on the PRO assessment checklist of the Cochrane collaboration and the criteria provided by Efficace for assessment of HRQL outcomes [Citation4]. The following criteria were evaluated: use of a PRO measurement instrument validated for use in cancer patients in general, preferably for the patient group and clinical situation in question, and report of baseline compliance, timing of PRO assessment and missing data. Studies that did not fulfil these criteria were considered as of insufficient standard. We also addressed their ability to give the reader an understandable presentation of the PRO and whether clinical significance was discussed.
The Cochrane collaboration's tool was used to assess the risk of bias in the RCTs [Citation10], including: concealment of allocation, sequence generation, blinding of outcome assessors, completeness of outcome data, and selective outcome reporting. For non-randomised trials, the completeness of the dataset, whether the primary outcome measure(s) were reliable and the quality of follow-up (frequency and length) were evaluated.
Results
Search results
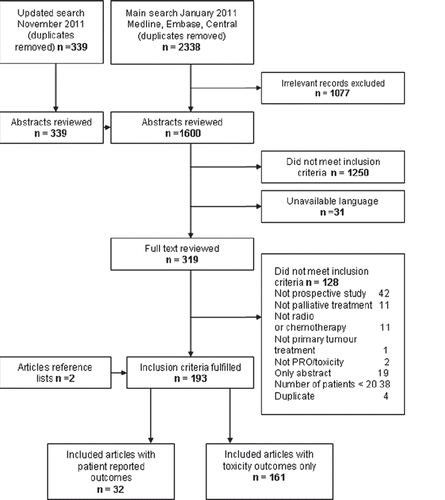
The search conducted in January 2011 resulted in 2338 records after removal of duplicates, of which 1077 records were not relevant (). The search was updated until November 2011 yielding 339 additional records. Of the 1600 abstracts reviewed, 1250 did not meet the inclusion criteria and 31 were excluded due to lack of relevant linguistic knowledge. The full text of 319 articles was reviewed, and 191 of these fulfilled the study inclusion criteria. Thirty publications included PROs in addition to toxicity and another two studies were retrieved from reference lists of identified articles; these are reviewed in the current paper.
Overview of the included studies
The 32 publications originating from 28 studies were classified according to the type of PRO () [Citation11–42]. Patient-reported dysphagia was part of HRQL assessments in 11 studies [Citation14,Citation15,Citation26,Citation27, Citation29–31,Citation34,Citation35,Citation40,Citation42] in addition to the seven publications reporting dysphagia only (). Only five studies were published before the year 2000 [Citation13,Citation21,Citation27,Citation41,Citation42]. The quality of the PRO reports was regarded to be of sufficient standard in 18 studies; the HRQL studies are listed in and (n = 14) [Citation12–15,Citation17–19,Citation26,Citation30–32,Citation40–42], and studies on patient-reported dysphagia only are listed in and (n = 4) [Citation16,Citation24,Citation28,Citation37].
Table I. Characteristics of patient-reported outcome (PRO) studies (n = 28).
Table II. Health related quality of life (HRQL) studies of sufficient standard (n = 14).
Table III. Outcome of health related quality of life (HRQL) studies of sufficient standard (n = 14).
Table IV. Patient-reported dysphagia studies of sufficient standard.
Table V. Outcome of patient-reported dysphagia studies of sufficient standard (n = 4).
Description of the HRQL studies
The majority of the HRQL studies evaluated palliative chemotherapy (n = 9) involving a wide range of regimens, with cisplatin and fluorouracil (CF) the most frequently used drugs (). Palliative radiotherapy was evaluated in five studies; stent vs. brachytherapy in two, external radiotherapy in two, and external radiotherapy with concomitant chemotherapy in a single study.
All except one of the HRQL studies used the European Organisation of Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ-C30) [Citation43]. In addition, the oesophagus module (EORTC QLQ-OES18 or QLQ-OES24) was used in six studies. One study used the Rotterdam symptom checklist [Citation44]. Reports of timing of assessment and the frequency of missing questionnaires varied between the studies. Baseline compliance ranged from 31% to 100%. The compliance during follow-up was more than 90% in one study in which patients received home visits by trained research nurses. For most of the studies, the attrition due to death or dropout for other reasons increased rapidly over time.
Some publications used tables and graphs to display the HRQL results [Citation12,Citation14,Citation17,Citation19,Citation25,Citation30], while others gave a short summary in text only [Citation18,Citation32,Citation40,Citation41]. Clinical significance was addressed in four of the studies [Citation14,Citation15,Citation17,Citation30].
Description of the dysphagia studies
The clinical intervention in the patient-reported dysphagia studies was multi-drug chemotherapy (n = 3) or radiotherapy with concomitant chemotherapy (n = 1) ().
There were small differences in the wording, but the dysphagia questions employed in each of the studies had a categorical response scale ranging from the ability to eat all food without problem to complete dysphagia. Baseline compliance was 100% in all studies, the number of patients was limited (n = 22 to 93) and compliance ranged from 63% to 100% for the second time point.
Treatment outcome
The main outcomes of the studies with sufficient standard on HRQL and patient-reported dysphagia are displayed in and .
As listed in , a significant difference in HRQL between treatment arms were reported in five of the eight RCTs. Docetaxel added to CF (DCF) improved HRQL compared to CF and epirubicin added to CF (ECF) resulted in longer preserved HRQL than its comparators; mitomycin + CF (MCF) and fluorouracil, doxorubicin and methotrexate (FAMTX) [Citation12,Citation32,Citation41]. Brachytherapy resulted in better HRQL compared to stent placement in two trials [Citation14,Citation25]. In the other three RCTs, no significant difference in the main HRQL outcome was reported [Citation18,Citation19,Citation31], but better physical functioning and less nausea/vomiting was reported for irinotecan combined with fluororouracil (IF) vs. CF [Citation19]. All phase II studies with HRQL as an outcome (n = 5) reported maintenance or some improvement in quality of life, and low toxicity of chemotherapy [Citation13,Citation17,Citation40] and of external radiotherapy [Citation15,Citation30]. Most of the papers (15/18) included PRO and toxicity results in their final conclusions.
Dysphagia after chemotherapy or chemoradiotherapy (treatments listed in ) was improved in three and stable in one of the four phase II studies with patient-reported dysphagia only [Citation16,Citation24,Citation28,Citation37] ().
Toxicity was usually reported by NCI-CTC, an older version of NCI-CTCAE [Citation7]. Nausea and diarrhoea were most frequent, peripheral neuropathy was reported after oxaliplatin-containing regimens, while hand-foot syndrome was found for capecitabine. Grade 3–4 toxicity and complications were infrequently observed.
The median overall survival ranged from 4.7 months (chemoradiotherapy) to 12.7 months (multi-drug chemotherapy) [Citation24,Citation37]. Only two RCTs found a survival benefit (8.9 months vs. 5.7 months, ECF vs. FAMTX and 9.2 vs. 8.6, DCF vs. CF) [Citation39,Citation41]. Tumour response was reported in all studies of chemotherapy.
Risk of bias
Eight of the sufficient standard PRO studies were RCTs and fulfilled the criteria of sequence generation and concealment of allocation, although most of them did not describe the randomisation procedure in detail. The baseline characteristics of the groups compared were balanced, and the risk of selection bias seemed to be low. Blinding of participants or personnel was not reported, while blinding of outcome assessors for tumour response was reported in three studies [Citation20,Citation31,Citation39]. Attrition is a possible source of bias, and differences in the dropout rates between the groups for PRO was either none (n = 3) [Citation12,Citation14,Citation25], small (n = 1) [Citation19] or not described (n = 4) [Citation18,Citation31,Citation32,Citation41]. Selective reporting bias was not found, and significant and non-significant differences between the intervention groups within a study were reported as required.
Discussion
In this review of treatment effects on palliative chemotherapy and radiotherapy in patients with advanced oesophageal cancer, only 32 of the records that fulfilled the inclusion criteria included PROs. This is worrying due to the lack of survival benefit for these treatments. Investigators need to question the clinical implications of a treatment when planning a study. In palliative studies, PROs are important endpoints, while tumour response may be irrelevant as long as survival is not improved.
The large heterogeneity between the studies made it difficult to draw conclusions regarding the effects of treatment on PROs. Surprisingly, more intensive chemotherapy gave better preservation of HRQL (DCF vs. CF) even though toxicity, in particular diarrhoea, was increased [Citation12,Citation39]. ECF resulted in better preserved global health scores than its comparator in two trials [Citation32,Citation41], and non-inferiority in one [Citation18]. These PRO results provided valuable data in addition to the evaluation of clinical response, and have had an impact on the extensive use of ECF as a reference treatment for advanced gastrooesophageal cancer, in particular, in Europe.
There was no clear association between HRQL and treatment toxicity. Two RCTs of chemotherapy reported better HRQL despite more treatment toxicity in the experimental arm vs. the standard arm [Citation12,Citation41], while the phase II studies reported improved HRQL and low levels of toxicity after treatment. Patients may tolerate toxicity to a greater extent when they have hope of treatment effect, and information about possible side effects may improve patient coping. There may also be publication bias; only one study reported negative results [Citation31].
It is a problem that all the RCTs on chemotherapy had mixed patient populations (gastric, oesophagogastric and oesophageal cancer). The results were reported for the whole group together, limiting the possibilities to obtain information relevant to patients with cancer of the oesophagus.
Even though radiotherapy is a commonly used palliative intervention for advanced cancer of the oesophagus [Citation1,Citation45], this was addressed in only nine of the 28 studies. However, the effect of palliative radiotherapy was confirmed. The effect of brachytherapy on overall HRQL was better than stent placement in two of the RCTs [Citation14,Citation25], and external radiotherapy provided relief from dysphagia for a substantial proportion of patients in non-randomised studies [Citation15,Citation24,Citation30]. The low proportion of studies on palliative radiotherapy is worrying. This could be due to lack of interest among investigators, or caused by the lack of funding for radiotherapy studies.
Although only a few clinical studies that included PROs were identified, it was encouraging that for most of them the PRO and toxicity results were included in their final conclusions and thereby enhanced the impact of these results on clinical decision-making.
On the other hand, caution should be taken using results from clinical trials to make conclusions regarding a treatment's impact on symptom relief and HRQL in clinical practise. Patients included in trials are carefully selected and may not be representative of a broader group of patients. Only one study included unselected patients from clinical practise, but unfortunately the palliative treatment applied was not specified [Citation42]. Further prospective observational studies of unselected patient populations receiving standard palliative treatment with PROs are warranted.
All but four studies were published in the last decade, confirming that PROs have only recently come to be recognised as valuable endpoints. Some research groups such as Cunningham's group at Royal Marsden Hospital, often include PRO [Citation13,Citation18,Citation31,Citation32,Citation41]. Even though guidelines are provided [Citation4,Citation5,Citation46], lack of motivation, resources and knowledge within research groups may be the reasons for not including PRO in most studies. On the other hand, we are pleased to acknowledge that authors such as Homs, Bergquist, O’Hanlon and Hayter have published papers using PRO as the primary endpoint [Citation14,Citation24,Citation25,Citation42]. In particular, Homs and co-workers managed both to conduct a study with a high quality of PRO methodology and to present the PRO results in a way that was easy to read [Citation25,Citation26].
All of the sufficient standard HRQL studies (n = 14) used questionnaires validated for cancer patients in general, but also for oesophageal cancer patients in particular. Although there is no consensus on which validated HRQL instrument to apply, the EORTC QLQ-C30 with or without diagnosis- specific modules, or the Functional Assessment of Chronic Illness Therapy cancer instrument (FACT-G) are recommended in order to facilitate comparisons between institutions and countries [Citation47].
In one study, one item was excluded from the chosen EORTC questionnaire because it was considered irrelevant to their patient group [Citation25,Citation26]. This is not recommended according to guidelines; the validity of the questionnaire may be compromised [Citation48].
High compliance is mandatory to obtain high quality of PRO data. Baseline compliance was above 80% for 15 of the 18 selected studies ( and ). A completed patient questionnaire as an inclusion criterion in a study ensures high baseline compliance. The involvement of a research nurse is crucial to achieve high compliance both at baseline and at the follow-up evaluations [Citation25,Citation49]. Most of the studies had a high attrition rate, but the reasons for dropouts were seldom reported. High attrition rate and low compliance are often recognised as potential sources of bias in results. A possible bias due to different dropout rates between the treatment arms was addressed in two studies [Citation12,Citation19]. However, a study by Ahlner-Elmquist did not find a difference in HRQL between participants and dropouts three months before death, and concluded that data from patients close to death may be representative of a larger group [Citation50].
To use tables and graphs to display the PRO results is an excellent way to give a concise and clear presentation of the HRQL from a number of subscales [Citation12,Citation25,Citation30]. It also simplifies and explains the results for the clinicians.
Some of the studies reported the HRQL results in text only, and a few of these only reported the global health status subscale [Citation18,Citation31]. Publications reporting the full results of a clinical trial with HRQL as a secondary endpoint often do not have the necessary space to report quality of life results. For clinical trials with a predefined HRQL endpoint, it is recommended that this should be reported together with the clinical endpoints in the main publication [Citation4]. However, all the subscales should be taken into account when reporting HRQL since it is well accepted that quality of life is multidimensional. A separate publication of the HRQL results was used by three studies [Citation12,Citation19,Citation20,Citation25,Citation26,Citation39]. This may limit the impact of the patients’ HRQL on clinical decisions [Citation51].
Only four of the studies addressed clinical significance. All of them referred to Osoba et al. who defined a difference of at least 10 points in the HRQL score after treatment, measured with EORTC QLQ-C30, as a clinically meaningful change [Citation52]. It is worrying that the rest of the studies rely on the level of statistical significance instead of interpreting the magnitude of clinical change.
For patients in a palliative situation, it is crucial to evaluate treatment effects on their symptoms and quality of life. In this review there were few identified papers with PROs as endpoints and high quality of PRO measurement. However, in the studies with PROs and sufficient methodology, brachytherapy, external radiotherapy and combination chemotherapy improved HRQL and dysphagia. Negative results were reported in one paper only, and one may wonder whether a publication bias could be present. The results from studies with mixed patient groups or where gastric cancer patients dominate should be interpreted with caution with regard to oesophageal cancer. In palliative studies, PROs are the most important measures of treatment effect when treatment does not prolong survival, and more relevant than tumour response. In future studies, a valid evaluation regarding treatment effects on patient- reported symptoms and HRQL should include PROs as predefined endpoints and with adequate methodology to ensure high compliance. Furthermore, attrition and clinical significance must be addressed.
Supplementary Appendix 1
Download PDF (175.4 KB)Acknowledgements
The authors are grateful for the advice given by M. Klemp of the Norwegian Knowledge Centre for the Health Services on the protocol writing and study design. We thank M. Isachsen and G. Kleven, the healthcare librarians who participated in the searches and obtained unavailable publications.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The research fellowship for Cecilie Delphin Amdal was provided by the Norwegian Extra Foundation for Health and Rehabilitation, who also provided funding for the project together with the Norwegian Cancer Society.
References
- Berger B, Belka C. Evidence-based radiation oncology: Ooesophagus. Radiother Oncol 2009;92:276–90.
- Homs MY, Gaast A, Siersema PD, Steyerberg EW, Kuipers EJ. Chemotherapy for metastatic carcinoma of the esophagus and gastro-esophageal junction. Cochrane Database Syst Rev 2006; 4:CD004063.
- Blazeby JM. Measurement of outcome. Surg Oncol 2001;10:127–33.
- Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D’haese S, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: A checklist for evaluating HRQOL outcomes in cancer clinical trials – does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol 2003;21:3502–11.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane collaboration 2009 Sep.
- Bottomley A, Jones D, Claassens L. Patient-reported outcomes: Assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur J Cancer 2009;45:347–53.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81.
- Blazeby JM, Alderson D, Winstone K, Steyn R, Hammerlid E, Arraras J, et al. Development of an EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. The EORTC Quality of Life Study Group. Eur J Cancer 1996;32A:1912–7.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–12.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928.
- Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, et al. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: Results of a phase II study. Cancer 2002;94:641–6.
- Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol 2007;25:3210–6.
- Bamias A, Hill ME, Cunningham D, Norman AR, Ahmed FY, Webb A, et al. Epirubicin, cisplatin, and protracted venous infusion of 5-fluorouracil for esophagogastric adenocarcinoma: Response, toxicity, quality of life, and survival. Cancer 1996;77:1978–85.
- Bergquist H, Wenger U, Johnsson E, Nyman J, Ejnell H, Hammerlid E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus 2005; 18:131–9.
- Burmeister BH, Walpole ET, Burmeister EA, Thomas J, Thomson DB, Harvey JA, et al. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol 2005;10:256–61.
- Cho SH, Chung IJ, Song SY, Yang DH, Byun JR, Kim YK, et al. Bi-weekly chemotherapy of paclitaxel and cisplatin in patients with metastatic or recurrent esophageal cancer. J Korean Med Sci 2005;20:618–23.
- Conroy T, Etienne PL, Adenis A, Ducreux M, Paillot B, Oliveira J, et al. Vinorelbine and cisplatin in metastatic squamous cell carcinoma of the oesophagus: Response, toxicity, quality of life and survival. Ann Oncol 2002;13:721–9.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46.
- Curran D, Pozzo C, Zaluski J, Dank M, Barone C, Valvere V, et al. Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: Results of a randomised phase III trial. Qual Life Res 2009;18:853–61.
- Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008;19:1450–7.
- Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 1994; 5:609–16.
- Frobe A, Jones G, Jaksic B, Bokulic T, Budanec M, Iva M, et al. Intraluminal brachytherapy in the management of squamous carcinoma of the esophagus. Dis Esophagus 2009;22: 513–8.
- Harbord M, Dawes RFH, Barr H, Giovannini M, Viens P, Eysselein V, et al. Palliation of patients with dysphagia due to advanced esophageal cancer by endoscopic injection of cisplatin/epinephrine injectable gel. Gastrointest Endosc 2002;56:644–51.
- Hayter CR, Huff-Winters C, Paszat L, Youssef YM, Shelley WE, Schulze K. A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol 2000;56: 329–33.
- Homs MY, Essink-Bot ML, Borsboom GJ, Steyerberg EW, Siersema PD, Dutch SIREC Study Group. Quality of life after palliative treatment for oesophageal carcinoma – a prospective comparison between stent placement and single dose brachytherapy. Eur J Cancer 2004;40:1862–71.
- Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: Multicentre randomised trial. Lancet 2004;364:1497–504.
- Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 1999;17: 3270–5.
- Jatoi A, Tirona MT, Cha SS, Alberts SR, Rowland KM, Morton RF, et al. A phase II trial of docetaxel and CPT-11 in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and gastric cardia. Int J Gastrointest Cancer 2002;32:115–23.
- Jatoi A, Murphy BR, Foster NR, Nikcevich DA, Alberts SR, Knost JA, et al. Oxaliplatin and capecitabine in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction and gastric cardia: A phase II study from the North Central Cancer Treatment Group. Ann Oncol 2006; 17:29–34.
- Kassam Z, Wong RK, Ringash J, Ung Y, Kamra J, DeBoer G, et al. A phase I/II study to evaluate the toxicity and efficacy of accelerated fractionation radiotherapy for the palliation of dysphagia from carcinoma of the oesophagus. Clin Oncol (R Coll Radiol) 2008;20:53–60.
- Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II study. Ann Oncol 2010;21: 2213–9.
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1996–2004.
- Rupinski M, Zagorowicz E, Regula J, Fijuth J, Kraszewska E, Polkowski M, et al. Randomized comparison of three palliative regimens including brachytherapy, photodynamic therapy, and APC in patients with malignant dysphagia (CONSORT 1a) (Revised II). Am J Gastroenterol 2011;106: 1612–20.
- Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009;104:1674–85.
- Spencer GM, Thorpe SM, Blackman GM, Solano J, Tobias JS, Lovat LB, et al. Laser augmented by brachytherapy versus laser alone in the palliation of adenocarcinoma of the oesophagus and cardia: A randomised study. Gut 2002;50:224–7.
- Sumpter K, Harper-Wynne C, Cunningham D, Rao S, Tebbutt N, Norman AR, et al. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer 2005;92:1976–83.
- Taeb J, Artru P, Baujat B, Mabro M, Carola E, Maindrault F, et al. Optimisation of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of hydroxyurea, leucovorin, 5-FU and cisplatin (HLFP regimen) for metastatic oesophageal cancer. Eur J Cancer 2002;38:661–6.
- Tebbutt NC, Norman A, Cunningham D, Iveson T, Seymour M, Hickish T, et al. A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Ann Oncol 2002;13:1568–75.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006; 24:4991–7.
- van Meerten E, Eskens FA, van Gameren EC, Doorn L, van der GA. First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: A phase II study. Br J Cancer 2007;96:1348–52.
- Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
- O’Hanlon DM, Harkin M, Karat D, Sergeant T, Hayes N, Griffin SM. Quality-of-life assessment in patients undergoing treatment for oesophageal carcinoma. Br J Surg 1995;82: 1682–5.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality- of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- de Haes JC, van Knippenberg FC, Neijt JP. Measuring psychological and physical distress in cancer patients: Structure and application of the Rotterdam Symptom Checklist. Br J Cancer 1990;62:1034–8.
- Gaspar LE, Nag S, Herskovic A, Mantravadi R, Speiser B. American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Clinical Research Committee, American Brachytherapy Society, Philadelphia, PA. Int J Radiat Oncol Biol Phys 1997;38:127–32.
- Young T, de Haes H, Curran D, Fayers P, Brandberg Y, Vanvoorden V, et al. Guidelines for assessing quality of life in EORTC clinical trials. Available from: http://groups.eortc.be/qol/manuals [cited 2012 Oct 3].
- Blazeby JM, Kavadas V, Vickery CW, Greenwood R, Berrisford RG, Alderson D. A prospective comparison of quality of life measures for patients with esophageal cancer. Qual Life Res 2005;14:387–93.
- Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. The EORTC QLQ-C30 Scoring Manual, 3rd ed. Available from: http://groups.eortc.be/qol/manuals [cited 2012 Oct 3].
- Blazeby JM, Nicklin J, Brookes ST, Winstone K, Alderson D. Feasibility of quality of life assessment in patients with upper gastrointestinal tract cancer. Br J Cancer 2003;89:497–501.
- Ahlner-Elmqvist M, Bjordal K, Jordhoy MS, Kaasa S, Jannert M. Characteristics and implications of attrition in health-related quality of life studies in palliative care. Palliat Med 2009;23:432–40.
- Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer 2008;44:1793–8.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.