Abstract
Background. Gastrointestinal stromal tumors (GISTs) can be effectively treated with tyrosine kinase inhibitors (TKIs). However, some patients with GIST develop drug resistance, and alternative treatment strategies are therefore needed. The aim of this study was to analyze the expression of somatostatin receptors (SSTR) in GIST as a target for peptide receptor-mediated radiotherapy (PRRT). Material and methods. Expression profiling of SSTR1–5 was performed on biopsies from 34 GISTs (16 gastric tumors, 15 small intestinal tumors, and three rectal tumors). SSTR scintigraphy (111In-octreotide) and measurement of 111In activity in tumor specimens was performed in seven patients. Uptake and internalization of 177Lu- octreotate was studied in primary cell cultures from two patients. Results. Quantitative PCR analysis showed expression of SSTR1 and SSTR2 in the majority of tumors, while SSTR3–5 were expressed at low levels. Immunohistochemical analysis confirmed the presence of SSTR1 and SSTR2 proteins in all GISTs, and SSTR3–5 in a subset of tumors. Diagnostic imaging by SSTR scintigraphy, using 111In-octreotide, demonstrated tumor uptake of 111In in three of six GIST patients. Measurement of 111In activity in excised tumor specimens from five patients gave tumor-to-blood (T/B) activity ratios of between eight and 96. Tumor cells in primary culture (gastric and small intestinal GIST) specifically bound and internalized 177Lu when incubated with the therapeutic compound 177Lu-octreotate for 4–48 hours (p < 0.05). Conclusion. Peptide receptor-mediated radiotherapy via SSTR may provide a novel treatment strategy in carefully selected GIST patients with TKI-resistant tumors.
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract. Mutations of KIT or PDGFRA are the primary events in tumor development and cause constitutive activation of the receptor tyrosine kinase (TK). Approximately 80% of all GISTs carry KIT mutations, while 5–8% have mutations in PDGFRA. About 12–15% of GISTs lack such mutations [Citation1]. Tumors carrying KIT or PDGFRA mutations can be successfully treated with the tyrosine kinase inhibitor (TKI) imatinib. The median overall survival of patients with GIST has improved markedly after the introduction of imatinib, which has become the primary treatment in patients with high-risk tumors [Citation2,Citation3]. Primary resistance to imatinib is seen in 10–15% of GIST patients and is related to the type of KIT or PDGFRA mutation harbored. Secondary resistance develops in 50–70% of patients after prolonged treatment [Citation4]. Sunitinib is used as second-line therapy after imatinib failure, but the duration of response is often limited [Citation5] emphasizing the need for alternative therapeutic strategies [Citation6]. Targeting of mTOR and IGF1R in wild-type and pediatric GIST are examples of such strategies [Citation7,Citation8].
With the identification of a neuroendocrine (NE) phenotype in GIST, e.g. expression of peptide receptors [Citation9,Citation10], peptide receptor-mediated radiotherapy (PRRT) may become a future treatment option. Somatostatin receptors (SSTRs) can be targeted by radiolabeled somatostatin analogs, as already shown for neuroendocrine tumors [Citation11]. There are five different receptor subtypes, SSTR1–5, which bind ligands with different affinities. SSTR2 is the most widely expressed SSTR subtype in NE tumors. Octreotide, a long-acting somatostatin analog, preferentially binds to SSTR2&5. NE tumor cells expressing SSTR2&5 bind and internalize radiolabeled octreotide and will thus deliver the radionuclide close to the nucleus of the tumor cell [Citation12].
The aim of this study was to determine whether SSTR-mediated radiotherapy could be a therapeutic option for a subgroup of GIST patients. Firstly, we characterized the SSTR expression profile in GISTs. Secondly, we explored whether diagnostic studies with 111In-octreotide could be helpful in localizing tumors. Finally, we quantitated the binding and uptake of the therapeutic compound 177Lu-octreotate both in vitro and in vivo.
Material and methods
Patients and tumor material
This retrospective study included 34 patients with GIST who had undergone surgery at the Department of Surgery, Sahlgrenska University Hospital, Gothenburg, Sweden, 1997–2008. The histopathological diagnosis was confirmed by typical morphology and positive staining for KIT and ANO1 (DOG1). For details on clinicopathological data see . The series included nine females and 25 males with a median age of 65 (range 10–82) years at the time of diagnosis. The location of the primary tumor was: stomach (n = 16), small intestine (n = 15), and rectum (n = 3). Twenty-two tumors were classified as high-risk, four as intermediate-risk, and eight as low-risk tumors according to the NIH consensus classification [Citation13]. Four patients were on neoadjuvant and two patients were on palliative treatment with imatinib at the time of surgery. Follow-up of recurrence-free survival (RFS) and overall survival (OAS) ended by December 2010. The median RFS was 38 months (range 0–131 months) and OAS was 63.5 months (range 15–552 months) following diagnosis [Citation14,Citation15].
Table I. Clinico-pathological and genetic characterization of 34 patients with gastrointestinal stromal tumor.
Ethics
For the use of clinical materials for research purposes, we obtained consent from the patients and approval from the Regional Ethical Review Board in Gothenburg, Sweden.
Quantitative real-time polymerase chain reaction (qPCR)
SSTR1–5 mRNA expression in fresh tumor biopsies obtained at surgery and primary cell cultures was analyzed by qPCR. Total RNA was isolated from biopsies and cell cultures using Trizol Reagent and RNeasy Mini Kit (Qiagen, Hilden, Germany) and this was followed by DNase treatment (DNA-free; Ambion Inc., Austin, TX, USA). cDNA was synthesized using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA). Primers and probes were purchased from Applied Biosystems: Hs00265617_s1 (SSTR1), Hs00990356_m1 (SSTR2), Hs01066399_m1 (SSTR3), Hs01566620_s1 (SSTR4), Hs00990407_s1 (SSTR5), and Hs99999903_m1 (ACTB). The samples were analyzed in triplicates and subjected to qPCR cycling conditions according to the manufacturer´s protocol (Applied Biosystems). The cycle threshold (Ct) of target genes and the housekeeping gene, ACTB, was determined. mRNA expression values for each target gene were calculated relative to the housekeeping gene as fold change per 1000 ACTB copies [1000x2-(Ct(target gene)-Ct(ACTB))].
Immunohistochemistry
All tumors were analyzed by immunohistochemistry for SSTR1–5 receptor protein. Formalin-fixed paraffin-embedded tumor specimens were subjected to immunohistochemical staining using Dako EnVision+ System HRP-labeled polymer according to the manufacturer's protocol (DakoCytomation, Glostrup, Denmark). The following primary antibodies were used: anti-SSTR1 [clone (15F10) 2D7, mouse monoclonal; Advanced Targeting Systems, San Diego, CA, USA], anti-SSTR2 (rabbit polyclonal; Human Protein Atlas, Sweden), anti-SSTR3 (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA), anti-SSTR4 (rabbit polyclonal; Novus Biologicals), and anti-SSTR5 (rabbit polyclonal; Thermo Fisher Scientific Inc., Waltham, MA, USA). Positive tissue controls were normal human cerebellum (for SSTR1, 2, 3 and 5) and human insulinoma (for SSTR4). Negative controls were sections incubated identically, except for the primary antibody, which was omitted. The extent of tumor labeling was scored by two independent observers. Tumor biopsies were categorized as being positive when labeling was confined to the cytoplasm and cell membrane of tumor cells, and scored into three groups (1 + score: between 10% and 25% of all tumor cells labeled; 2 + score: between 25% and 75% of all tumor cells labeled; and 3 + score: more than 75% of all tumor cells labeled). Biopsies with less than 10% labeling of tumor cells were categorized as negative.
Immunocytochemistry and confocal laser microscopy
GIST tumor cells (see below) were grown on collagen-coated chamber slides (8-well Biocoat® CultureSlide; BD Biosciences, Bedford, MA, USA). Tumor cells were fixed in 4% buffered paraformaldehyde and incubated with primary antibodies directed against KIT (rabbit polyclonal; Dako), DOG-1 (clone K9, mouse monoclonal; Novocastra™ Leica Microsystems GmbH, Wetzlar, Germany), CD34 (clone QBEnd10, mouse monoclonal; Dako), and SSTR1–5 (see above). As secondary antibodies, we used goat anti-mouse (Alexa Fluor 488-conjugate; Molecular Probes Inc., Eugene, OR, USA) and goat anti-rabbit (Alexa Fluor 594-conjugate; Molecular Probes Inc.). Tumor cells incubated identically served as controls, except that the primary antibody was omitted. DAPI was used as nuclear counterstaining. Tumor cells were examined with confocal microscopy using a Zeiss LSM 510 META system.
Tumor cell cultures
Tumor biopsies from two patients with GIST (patients nos. 15 and 25) were obtained at surgery. Biopsies were enzymatically dissociated using collagenase and DNase before tissue culture. The tumor cells were seeded onto collagen-coated, 24-well tissue culture plates (Biocoat® Multiwell Plate; BD Biosciences) at a density of 750 000 cells per well and incubated at 37°C in 250 μl RPMI 1640 medium supplemented with 10% fetal calf serum, L-glutamine, and PEST in a humidified atmosphere with 5% CO2. The experiments were performed on tumor cells after four days in primary culture. Cell cultures consisted of approximately 80–90% tumor cells.
Radiopharmaceuticals
Radiolabeling of DOTA-Tyr3-octreotate with 177Lu (177Lu-octreotate) and of DTPA-D-Phe1-octreotide with 111In (111In-octreotide) (OctreoScan®) was performed according to the instructions of the manufacturers (NRG, Petten, the Netherlands and Tyco Healthcare, Mallinckrodt, St Louis, MO, USA, respectively). Chromatography of radiopharmaceuticals was performed using instant thin layer chromatography (ITLC-SG) (Gelman Instrument Company, Ann Arbor, MI, USA) with 0.1 M sodium-citrate (pH 5) as the mobile phase. The fractions of peptide-bound 177Lu and 111In were shown to be more than 98% and 99%, respectively.
177Lu-octreotate binding to cultured tumor cells
Cultured tumor cells were incubated with 250 μl medium containing 177Lu-octreotate, corresponding to 10 nM of octreotate (approximately 8–9 kBq/well), at 37°C for 4, 24, and 48 hours (four replicates for 4 and 24 hours, and eight replicates for 48 hours). Tumor cells incubated with 250 μl medium containing 10 nM 177Lu-octreotate supplemented with 5 μM octreotide (Sandostatin; Novartis, Basel, Switzerland) served as controls. Otherwise, the controls were treated identically. At the end of the experiment, the medium was removed and saved for measurement of 177Lu activity (unbound 177Lu). The cells were subsequently washed with Ca- and Mg-free PBS and incubated with 0.5 ml 0.05% trypsin solution (cat. no. 15090046; Invitrogen) at 37°C for 15 minutes. The trypsin/cell medium was transferred to plastic tubes and centrifuged at 900 × g for 5 minutes. The supernatant was saved for measurement of 177Lu activity (surface-bound 177Lu). The cell pellets were washed with PBS and saved for measurement of 177Lu activity (internalized 177Lu). The 177Lu activity in each sample was determined with a Wallac 1480 gamma counter (WIZARD™ 3”; Wallac Oy, Finland) with a 10% energy window over the 208 keV photon peak. At least 1000 counts for each sample were collected to obtain low statistical uncertainties. Corrections were made for physical decay, background, and dead time.
111In-octreotide scintigraphy and 111In activity concentration in tumor samples
Seven GIST patients (nos. 8, 19, 25, 29, 31, 32, and 34) received 170–240 MBq 111In-DTPA-D- Phe1-octreotide (111In-octreotide) by intravenous injection. Scintigraphy was performed in six of the patients at around 24 hours after injection of 111In-octreotide using a gamma camera (General Electric 400 AC/T; General Electric, London, UK). Static anterior and posterior images from the base of the skull to the pelvis were acquired for all patients. One patient was not analyzed by scintigraphy due to logistic reasons. Surgery was performed 2–22 days after the injection of 111In-octreotide. In five patients, tumor samples together with blood samples were drawn during surgery. Samples were weighed, and 111In activity in each sample was measured with a Wallac 1480 gamma counter (WIZARD™ 3”). The 111In activity concentration in a tissue sample was determined as the fraction of injected 111In activity per unit mass of that tissue sample (corrected for decay in radioactivity to the time of injection), and given as %IA/g. The tumor-to-blood 111In activity concentration ratio (T/B) was calculated.
Statistics
The binding efficiency between 177Lu-octreotate-incubated cell cultures and controls was tested by robust linear regression in the statistical language R version 2.13.0, using the MASS package.
Results
GISTs regularly express SSTR1 and SSTR2
SSTR subtypes 1 and 2 were detected in 33/34 biopsies analyzed by qPCR. SSTR1 showed higher mRNA values than SSTR2 (target copies per 1000 ACTB; median for SSTR1: 0.40; and median for SSTR2: 0.33). However, there was large variation in the expression of these two receptor subtypes, which was reflected by their mean values and ranges [SSTR1: mean 54 (range 0.00–445); and SSTR2: mean 0.62 (range 0.00–3.26)] (). For Ct and ΔCt [Ct(target gene) – Ct(ACTB)] values, see Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.733075. SSTR3–5 was detected in 6–41% of tumors. The SSTR mRNA profile of GIST was different from that of NE tumors (medullary thyroid carcinoma, midgut carcinoid) with lower expression of SSTR2&5 in GIST (). Immunohistochemical analysis showed that all tumors were positive for SSTR1 and SSTR2, while positive labeling for SSTR3–5 was detected in 0–29% (). Labeling for SSTR1, 2, 3, and 5 was observed over tumor cell cytoplasm (). Weak labeling for SSTR1, 2, 3, and 5 was also observed over stroma and blood vessels. Immunocytochemical analysis of cultured tumor cells confirmed predominantly cytoplasmic localization of SSTR1, 2, 3, and 5 in tumor cells, while SSTR4 was negative ().
Figure 1. (A) Comparison of tumor-to blood activity concentrations between patients with GIST and NE tumors, i.e. medullary thyroid carcinoma (MTC) and midgut carcinoids (MC). The ratios are classified as groups dependent on disease type and time after injection. Data on T/B values in MTC and MC were from Forssell-Aronsson et al. [Citation18]. In general MC had higher ratios than MTC (biopsied according to the same time scale). On the other hand, GIST were biopsied according to a protracted time scale making direct comparison difficult. However, the GIST patient with highest T/B ratio (96) long time after injection (13 days) seemed to be suitable for PRRT. (B) Comparison of SSTR mRNA values in GIST tumors (n = 34) compared to NE tumors (MTC, n = 5; MC, n = 12). MTC and MC showed high expression of SSTR2&5 (with high affinity for octreotide/octreotate), while GIST have much lower expression of these two receptor subtypes.
![Figure 1. (A) Comparison of tumor-to blood activity concentrations between patients with GIST and NE tumors, i.e. medullary thyroid carcinoma (MTC) and midgut carcinoids (MC). The ratios are classified as groups dependent on disease type and time after injection. Data on T/B values in MTC and MC were from Forssell-Aronsson et al. [Citation18]. In general MC had higher ratios than MTC (biopsied according to the same time scale). On the other hand, GIST were biopsied according to a protracted time scale making direct comparison difficult. However, the GIST patient with highest T/B ratio (96) long time after injection (13 days) seemed to be suitable for PRRT. (B) Comparison of SSTR mRNA values in GIST tumors (n = 34) compared to NE tumors (MTC, n = 5; MC, n = 12). MTC and MC showed high expression of SSTR2&5 (with high affinity for octreotide/octreotate), while GIST have much lower expression of these two receptor subtypes.](/cms/asset/2460d324-ee3c-4fcd-a1d9-92cb792e6c0f/ionc_a_733075_f0001_b.gif)
Table II. Expression profiles of somatostatin receptors in gastrointestinal stromal tumors. Biopsies from 34 patients with GIST were analyzed by real-time qPCR and IHC. qPCR values are expressed as fold change of target gene per 1000 ACTB copies. IHC labeling was scored as the number of positive tumor cells: - = no labeling, 1+ = 10–25%, 2+ = 25–75%, 3+ = > 75%.
Figure 2. Expression of somatostatin receptor protein in GIST. SSTR1, 2, 3, and 5 proteins localized to tumor cell cytoplasm. SSTR4 protein was undetectable. Immunoperoxidase staining of consecutive sections from tumor biopsy from patient no. 12 (small intestinal, low-risk, wt in KIT and PDGFRA). Brown = antibody labeling. Blue = nuclear counter staining. Left panel (A, C, E, G, I): low power (bar equals 50 μm). Right panel (B, D, F, H, J): high power (bar equals 20 μm). *indicates luminal side of vascular profile.
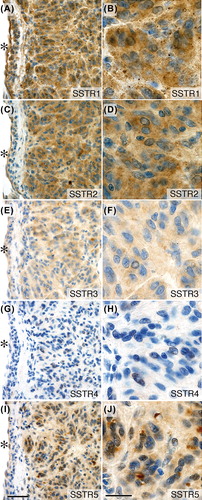
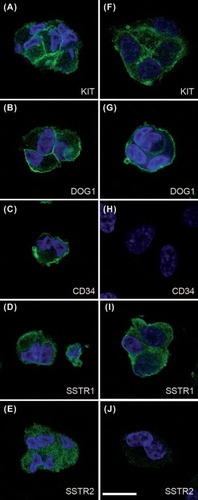
Figure 3. GIST in primary culture. Tumor cells were positive for the GIST markers KIT and DOG1 (ANO1). The marker CD34 was positive in one tumor. Tumor cells also expressed SSTR1 and 2 with membrane and cytoplasmic labeling. (A–E) Patient no. 15 (gastric, low-risk, PDGFRA exon 18 (D842V) missense mutation). (F–J) Patient no. 25 (small intestinal, high-risk, KIT exon 11 (P573_Y578) duplication mutation). Immunofluorescent labeling and confocal laser microscopy; antibody labeling in green color and DAPI-stained nuclei in blue color. Bar equals 20 μm.
GIST tumors accumulate 111In-octreotide and can be visualized by scintigraphy
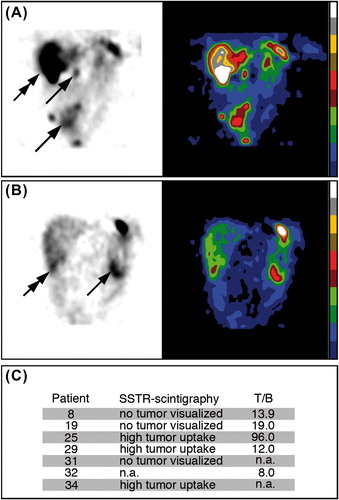
Six patients were subjected to SSTR scintigraphy preoperatively (nos. 8, 19, 25, 29, 31, and 34). Positive tumor imaging was obtained in three patients (nos. 25, 29, and 34) (). In five patients, the 111In activity concentration was studied in excised tumor biopsies. The T/B activity concentration ratio was 8.0–96 (). The T/B values in GIST seemed to be lower than in NE tumors (medullary thyroid carcinoma, carcinoids) ().
Figure 4. SSTR scintigraphy and tumor-to-blood 111In activity concentration ratios (T/B) in tumor biopsies from GIST patients injected with 111In-DTPA-D-Phe1-octreotide. (A) Tumor imaging of patient no. 25 with primary small intestinal GIST non-radically resected, and multiple abdominal (arrows) and liver (double arrow) recurrences four years after primary surgery. The primary tumor had KIT exon 11 duplication mutation and responded to imatinib. (B) Tumor imaging of patient no. 29 with primary GIST close to the ligament of Treitz (arrow) and multiple liver metastases in the right liver lobe (double arrow). This patient was wt for KIT and PDGFRA and resistant to imatinib. The patient survived for only two years after surgery. (C) Summary of observations at SSTR-scintigraphy and maximal T/B values in GIST patients.
GIST cells in primary culture bind and internalize 177Lu–octreotate
GIST in primary cell culture specifically bound 177Lu when incubated with 177Lu-octreotate (). The amount of surface-bound 177Lu increased with incubation time (p < 0.05). In control cultures, surface-binding of 177Lu was reduced by 80–82% at 48 hours, when excess unlabeled octreotide was added. GIST cells also internalized 177Lu after incubation with 177Lu-octreotate. The amount of internalized 177Lu increased continuously during the incubation period (p < 10215). In control cultures, internalization of 177Lu was reduced by 98–99% at 48 hours when unlabeled octreotide was added. The relative amount of internalized vs. surface-bound 177Lu increased from 1.8–2.5 at 4 hours to 7.0–9.8 at 48 hours. The primary cell cultures from gastric GIST (patient no. 15) and small intestinal GIST (patient no. 25) bound and internalized 177Lu to the same degree ( and Supplementary Table 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.733075.
Figure 5. Binding and internalization of 177Lu-DOTA-Tyr3-octreotate in cultured GIST cells. Tumor cells were cultured with 177Lu-octreotate (10 nM) with or without unlabeled octreotide (5 μM). The fraction of unbound, surface-bound, and internalized 177Lu in GIST tumor cells was measured after 4, 24, and 48 hours. Values represent mean of 4–8 replicates. Error bars are smaller than symbols. Internalization of 177Lu was significantly higher in 177Lu-octreotate-incubated tumor cells than in controls (p < 10-15). The amount of internalized 177Lu increased steadily during the incubation period. (A–C) Patient no. 15 (gastric, low-risk, PDGFRA exon 18 (D842V) missense mutation; SSTR1/2 mRNA values: 107/0.38). (D–F) Patient no. 25 [small intestine, high-risk, KIT exon 11 (P573_Y578) duplication mutation; SSTR1/2 mRNA values: 445/0.60; clearly positive imaging; T/B: 96]. Further statistical details are presented in Supplementary Table 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.733075.
![Figure 5. Binding and internalization of 177Lu-DOTA-Tyr3-octreotate in cultured GIST cells. Tumor cells were cultured with 177Lu-octreotate (10 nM) with or without unlabeled octreotide (5 μM). The fraction of unbound, surface-bound, and internalized 177Lu in GIST tumor cells was measured after 4, 24, and 48 hours. Values represent mean of 4–8 replicates. Error bars are smaller than symbols. Internalization of 177Lu was significantly higher in 177Lu-octreotate-incubated tumor cells than in controls (p < 10-15). The amount of internalized 177Lu increased steadily during the incubation period. (A–C) Patient no. 15 (gastric, low-risk, PDGFRA exon 18 (D842V) missense mutation; SSTR1/2 mRNA values: 107/0.38). (D–F) Patient no. 25 [small intestine, high-risk, KIT exon 11 (P573_Y578) duplication mutation; SSTR1/2 mRNA values: 445/0.60; clearly positive imaging; T/B: 96]. Further statistical details are presented in Supplementary Table 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.733075.](/cms/asset/571ef07c-26dc-487f-a998-e4a101fdfef1/ionc_a_733075_f0005_b.gif)
Discussion
GISTs are successfully treated with surgery and imatinib, resulting in markedly prolonged patient survival. However, resistance to imatinib is an increasing clinical problem, and alternative treatment strategies are therefore needed. Targeted radiotherapy via SSTR may be such an alternative treatment option in carefully selected patients. In this study, we explored the prerequisites for successful PRRT in GIST by examining the expression profile of SSTR in tumors and also binding and uptake of radiolabeled somatostatin analogs in vitro. All GISTs examined expressed SSTR, as determined by qPCR and immunohistochemistry. SSTR1 and 2 were the most abundant receptor subtypes, being present in more than 97% of all tumors analyzed by qPCR, while SSTR3–5 were detected at lower frequencies. Differences in the expression of SSTRs determined by qPCR and immunohistochemistry were noted (). Our results extend the observations made by Palmieri et al., who demonstrated SSTR expression in a small group of GISTs by immunohistochemistry [Citation10]. In the present study, we further tested the possibility of visualizing SSTR-expressing tumors by scintigraphy. SSTR scintigraphy was performed in six patients, with all tumors being visualized clearly in three of the patients. Measurements of 111In activity concentration in excised tumor specimens confirmed that there was specific uptake of radionuclide in tumor tissue with T/B values ranging from 8 to 96. The maximum T/B values in GIST were compared to those in medullary thyroid carcinoma and midgut carcinoids at various intervals after injection of radionuclide (). T/B values in GIST seemed to be in the same range as in medullary thyroid carcinoma but in general lower than in midgut carcinoids. 177Lu-octreotate treatment via SSTR has been given in few patients with differentiated thyroid carcinomas. Single patients showed partial tumor response [Citation16]. The patient (no. 25) with the highest T/B value (96) had multiple tumors, all visualized by SSTR scintigraphy. Binding and internalization of radiolabeled somatostatin analog was studied in tumor cells from two patients (nos. 15 and 25). Both experiments showed specific binding and internalization with rapid and continuous accumulation of radionuclide. Patient no. 25 underwent adjuvant imatinib treatment and developed a late non-resectable recurrence. He could have been a candidate for palliative PRRT. Patient no. 15 was recurrence-free two years after surgery and adjuvant treatment with imatinib/sunitinib. Considering the high degree of internalization of 177Lu in this patient, he might have been suitable for PRRT in case of non-resectable recurrence or TKI resistance. Of nine patients with primary resistance, four had SSTR1 mRNA values > 10 (nos. 12, 14, 15, and 31). These tumors had low values of SSTR2 (). Of the four patients with secondary resistance (nos. 3, 17, 22, and 33), only one (no. 17) had a high SSTR1 mRNA value (90.1). All these tumors had low SSTR2 values and two tumors had low SSTR5 values (). For one of the patients with resistance (no. 15), binding and internalization of 177Lu-octreotate were demonstrated in vitro.
A comparison between GISTs and NE tumors showed similarities and differences in SSTR expression and binding of radiolabeled somatostatin analogs (). Both kinds of tumors express multiple SSTR subtypes: GISTs mainly SSTR1&2 and carcinoid tumors mainly SSTR2&5 [Citation17]. The majority of NE tumors are imaged by 111In-octreotide scintigraphy due to preferential binding of octreotide to SSTR2&5. Heterogeneity among tumor lesions in the same patient, i.e. marked differences in 111In-octreotide uptake, is common in patients with NE tumors [Citation18]. Our data on SSTR1&2 expression indicate tumor heterogeneity in GIST, e.g. good tumor visualization in patients nos. 29 and 34 was obtained despite low SSTR expression values in the analyzed biopsies ( and ) [Citation18]. The uptake of radiolabeled somatostatin analog in cultured GIST cells was characterized by a high specific binding and internalization, similar to that described for NE tumors [Citation12].
Our experimental results show that there might be suitable conditions for PRRT via SSTR in certain GIST patients with TKI resistance. Currently available radiolabeled somatostatin analogs (e.g. 111In-octreotide, 177Lu-octreotate, and 90Y- octreotide) need to be evaluated for tumor specificity and toxicity, but would still not be optimal for therapy of GIST. Instead, the radiolabeled somatostatin analog should be optimized for this tumor type. The fact that SSTR1 is expressed at higher levels than SSTR2 in most GISTs, suggests that an analog with preferential binding to SSTR1, or to both SSTR1&2, should be tried. Octreotide and lanreotide have much higher affinity for SSTR2 than SSTR1, while the multi-receptor ligand pasireotide binds to SSTR1&2 with approximately the same affinity [Citation19], but radiolabeled pasireotide is not yet available. Before clinical trials with radiolabeled somatostatin analogs can be initiated, further studies on biodistribution and dosimetry of radionuclides are needed. If designing improved radiolabeled somatostatin analogs for GIST, one must consider both differences in binding affinities and internalization between receptor subtypes. The high uptake of 111In and 177Lu observed in this study most likely represents internalization of radiolabeled analog after binding to SSTR2&5. Experimental studies on human SSTR1–5 in transfected CHO-K1 cells showed maximal internalization of SSTR3, followed by SSTR5, 4, and 2 in decreasing order. No internalization was observed for SSTR1 [Citation20]. On the other hand, transfected HEK cells expressing rat SSTR of subtypes 1–5 showed a different pattern of receptor internalization, with maximal internalization of SSTR1–3 [Citation21]. The reported differences in SSTR1 receptor internalization are probably due to species differences and specific tumor types.
In conclusion, GISTs express multiple SSTR subtypes that can bind and internalize radiolabeled somatostatin analogs. The experimental data suggest that SSTR may be used for targeted radiotherapy in selected patients with non-resectable GIST and TKI resistance. Optimization of radiolabeled analogs and biodistribution and dosimetry studies will be needed before clinical trials of PRRT via SSTR can be started in GIST patients.
Tables 1–2
Download PDF (575.4 KB)Acknowledgements
The expert technical assistance of Gülay Altiparmak, Malin Berntsson, Pauline Brattberg, Ann-Christine Illerskog, Linda Inge, Lilian Karlsson, Ann Wikström, and Milan Lomsky is greatly appreciated. This study was supported by the Swedish Cancer Society, the Swedish Research Council, Sahlgrenska Academy (the government ALF agreement), the Johan Jansson Foundation for Cancer Research, the Assar Gabrielsson Research Foundation, the Sahlgrenska University Hospital Research Foundation, and the Royal Society of Arts and Sciences in Gothenburg.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 2008;3:557–86.
- Bümming P, Andersson J, Meis-Kindblom JM, Klingenstierna H, Engström K, Stierner U, et al. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: A centre-based study of 17 patients. Br J Cancer 2003;89:460–4.
- Van Glabbeke MM, Owzar K, Rankin C, Simes J, Crowley J, editors. GIST Meta-analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of gastrointestinal stromal tumors (GIST): A meta-analysis based on 1640 patients. 2007 ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007;25(18S):A10004.
- Wang WL, Conley A, Reynoso D, Nolden L, Lazar AJ, George S, et al. Mechanisms of resistance to imatinib and sunitinib in gastrointestinal stromal tumor. Cancer Chemother Pharmacol 2011;67(Suppl 1):S15–24.
- Younus J, Verma S, Franek J, Coakley N. Sunitinib malate for gastrointestinal stromal tumour in imatinib mesylate- resistant patients: Recommendations and evidence. Curr Oncol 2010;17:4–10.
- Nilsson B, Nilsson O, Ahlman H. Treatment of gastrointestinal stromal tumours: Imatinib, sunitinib – and then?Expert Opin Investig Drugs 2009;18:457–68.
- Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA 2008;105:8387–92.
- Pantaleo MA, Nicoletti G, Nanni C, Gnocchi C, Landuzzi L, Quarta C, et al. Preclinical evaluation of KIT/PDGFRA and mTOR inhibitors in gastrointestinal stromal tumors using small animal FDG PET. J Exp Clin Cancer Res 2010;29:173.
- Reubi JC, Korner M, Waser B, Mazzucchelli L, Guillou L. High expression of peptide receptors as a novel target in gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging 2004;31:803–10.
- Palmieri G, Montella L, Aiello C, Barbieri F, Di Vizio D, Schulz S, et al. Somatostatin analogues, a series of tissue transglutaminase inducers, as a new tool for therapy of mesenchimal tumors of the gastrointestinal tract. Amino Acids 2007;32:395–400.
- Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 2010;17:R53–73.
- Andersson P, Forssell-Aronsson E, Johanson V, Wängberg B, Nilsson O, Fjälling M, et al. Internalization of indium-111 into human neuroendocrine tumor cells after incubation with indium-111-DTPA-D-Phe1-octreotide. J Nucl Med 1996;37:2002–6.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33: 459–65.
- Arne G, Kristiansson E, Nerman O, Kindblom LG, Ahlman H, Nilsson B, et al. Expression profiling of GIST: CD133 is associated with KITexon 11 mutations, gastric location and poor prognosis. Int J Cancer 2011;129:1149–61.
- Bümming P, Nilsson B, Sörensen J, Nilsson O, Ahlman H. Use of 2-tracer PET to diagnose gastrointestinal stromal tumour and pheochromocytoma in patients with Carney triad and neurofibromatosis type 1. Scand J Gastroenterol 2006;41:626–30.
- Teunissen JJ, Kwekkeboom DJ, Krenning EP. Staging and treatment of differentiated thyroid carcinoma with radiolabeled somatostatin analogs. Trends Endocrinol Metab 2006; 17:19–25.
- Nilsson O, Kölby L, Wängberg B, Wigander A, Billig H, William-Olsson L, et al. Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours. Br J Cancer 1998;77:632–7.
- Forssell-Aronsson E, Bernhardt P, Nilsson O, Tisell LE, Wängberg B, Ahlman H. Biodistribution data from 100 patients i.v. injected with 111In-DTPA-D-Phe1-octreotide. Acta Oncol 2004;43:436–42.
- Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res 2010;29:19.
- Hukovic N, Panetta R, Kumar U, Patel YC. Agonist-dependent regulation of cloned human somatostatin receptor types 1-5 (hSSTR1-5): Subtype selective internalization or upregulation. Endocrinology 1996;137:4046–9.
- Roth A, Kreienkamp HJ, Nehring RB, Roosterman D, Meyerhof W, Richter D. Endocytosis of the rat somatostatin receptors: Subtype discrimination, ligand specificity, and delineation of carboxy-terminal positive and negative sequence motifs. DNA Cell Biol 1997;16:111–9.
