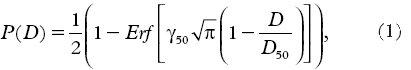Abstract
Purpose. The aim of this study was to investigate what bowel organ and delivered dose levels are most relevant for the development of ‘emptying of all stools into clothing without forewarning’ so that the related dose-responses could be derived as an aid in avoiding this distressing symptom in the future. Material and methods. Of the 77 gynecological cancer survivors treated with radiotherapy (RT) for gynecological cancer, 13 developed the symptom. The survivors were treated between 1991 and 2003. The anal-sphincter region, the rectum, the sigmoid and the small intestines were all delineated and the dose-volume histograms were exported for each patient. The dose-volume parameters were estimated fitting the data to the Relative Seriality (RS), the Lyman and the generalized Equivalent Uniform Dose (gEUD) model. Results. The dose-response parameters for all three models and four organs at risk (OARs) were estimated. The data from the sigmoid fits the studied models best: D50 was 58.8 and 59.5 Gy (RS, Lyman), γ50 was 1.60 and 1.57 (RS, Lyman), s was 0.32, n was 0.13 and a was 7.7 (RS, Lyman, gEUD). The estimated volume parameters indicate that the investigated OARs behave serially for this endpoint. Our results for the three models studied indicate that they have the same predictive power (similar LL values) for the symptom as a function of the dose for all investigated OARs. Conclusions. In our study, the anal-sphincter region and sigmoid fit our data best, but all OARs were found to have steep dose-responses for ‘emptying of all stools into clothing without forewarning’ and thus, the outcome can be predicted with an NTCP model. In addition, the dose to the four studied OARs may be considered when minimizing the risk of the symptom.
The number of gynecological cancer patients surviving is increasing rapidly and a significant number of these women have received external beam radiation therapy. Some of these women develop radiation- induced symptoms in the pelvic region. One of these symptoms, ‘emptying of all stools into clothing without forewarning’, is neither a fecal incontinence nor a pure urgency symptom. However, it is more related to urgency than to incontinence. More specifically this endpoint is associated with a decreased sensitivity that entails not being able to sense the need to go to the toilet and defecate and it also includes an irritative element that is responsible for the sudden emptying of a large volume of stools. We have previously described atomized symptoms by using the survivors’ own words, for this endpoint [Citation1]. This symptom severely decreases the quality of life of gynecological cancer survivors after pelvic radiotherapy and it has not been known which of the bowel organs with which the symptom is most strongly correlated.
In 2006, we performed a population-based matched control study, including 616 survivors treated with radiation therapy and 344 non- irradiated women. A study-specific, postal questionnaire with 351 questions covered physical symptoms from gastrointestinal, urinary and genital tracts, the pelvic bones, lower abdomen and legs. Additional information about the incidence, prevalence, intensity and duration of the symptoms and their impact on different aspects of social functioning was assessed [Citation2]. The highest age adjusted RR (12.7 95% CI 4.0–40.3) was found for having the symptom ‘emptying of all stools into clothing without forewarning’ at least once during the preceding six months, with a prevalence of 12% among the survivors compared to 0.9% among the non-irradiated control women [Citation3]. In a previous work, we reported on the relationship between mean absorbed dose to gastrointestinal OARs and the occurrence of ‘emptying of all stools into clothing without forewarning’ among 519 survivors for whom we had information on detailed dosimetric parameters [Citation4]. It was found that the mean absorbed dose to the bowel organs and the anal-sphincter region was related to the symptom.
For organ-symptom pairs where a clear dose- response relation is found, simple straightforward guidelines can be formulated and used to minimize the risks of future severe complications among cancer survivors. Depending on whether the organ behavior is serial or parallel the criteria can be formulated in terms of mean or maximum doses to the organ, respectively [Citation5]. A more powerful approach would be to determine dose-response parameters for the different Normal Tissue Complication Probability (NTCP) from clinical studies [Citation6]. The aim of this study was to determine the most relevant combination of OARs: the anal-sphincter region, the rectum, the sigmoid and the small intestines and the dose for the symptom ‘emptying of all stools into clothing without forewarning’ in order to derive the corresponding dose-volume response relationship as an aid for avoiding the symptom in the future.
Material and methods
Patient material
A patient cohort consisting of 519 survivors previously treated with RT for gynecological cancer, either at the Karolinska University Hospital Stockholm, or the Sahlgrenska University Hospital Gothenburg, during the period from 1991 to 2003 was investigated. To minimize any selection-induced problem in this group, we used the Swedish personal identity numbers and official population-based registers. Due to the lack of 3-D dose information in brachytherapy treatment plans, 83 survivors who did not receive any brachytherapy were selected in the first place. A preliminary multivariate analysis was performed indicating that except from the dose to the sigmoid, heart failure was a statistically significant risk factor. Since there were only six survivors with heart failure they were excluded. Therefore, 77 survivors were finally included and 13 of those experienced the investigated symptom. Different combinations of surgery and chemotherapy were used for different patients. The three criteria for the cancer survivors at follow-up in January 2006 were: born 1927 or later, able to read and understand Swedish and having no recurrent disease as has been described in detail in a previous paper [Citation3]. The study was approved by the Regional Ethics Committee.
Treatment planning and delineation
The predominant RT treatment technique before 1996 was two opposing fields, while after 1996 it was more common to use a four-field box technique. The treatment planning were performed by TMS (Nucletron, Veenendaal, the Netherlands) in Stockholm and Cadplan and Eclipse (Varian Medical Systems, Palo Alto, USA) in Gothenburg using 6–50 MV according to ICRU 1993 [Citation7]. Computed tomography (CT) (slice thickness: 5–20 mm) scanning was made with the patients in treatment position on a flat table, using laser markers and conversion factors to electron density. The fractionation schedules used during the treatment period were 1.6 Gy, 1.8 or 2.0 Gy. The prescribed dose was 39.6–46.0 Gy for endometrial cancer, 50.4 Gy for uterine sarcomas, 55.0–0.0 Gy for cervical cancer and for ovarian and fallopian tube the prescribed dose was 20.0 Gy to the abdomen and an additional 20.0 Gy to a volume with lowered cranial margin.
The four OARs were delineated in each treatment planning systems. The ‘anal-sphincter region’ was defined as the inner muscle layer of the sphincter up to the anal verge. The ‘rectum’ was extended from the anal verge to where it deviated from its mid position. The delineation of the ‘sigmoid colon’ started cranially from this point to where it could be located in the left part of the abdomen connecting to the colon descendens. For the ‘small intestines’ we delineated all visible small bowels in the pelvic cavity caudally of the sacroiliac joints. The delineation procedures have been described in detail in one of our previous papers [Citation4].
Evaluation
Questionnaire information was stored through Epidata (www.epidata.dk) and incorporated into SAS (version 9.2, SAS Institute Inc., Cary, NC, USA). The cumulative DVHs for the studied OARs were produced. For small intestines the absolute volume was used in the DVHs, since a part of the organ was delineated, while for the rest of the organs we used the relative volume. During the study period, different fractionation schedules have been used and the dose distributions in the OARs were non-uniform, therefore the doses were corrected to 2 Gy per fraction using the Linear-Quadratic model using an α/β ratio of 3 Gy. The parameters were estimated for the Relative Seriality (RS) [Citation8], the Lyman [Citation9,Citation10] and the generalized Equivalent Uniform Dose (gEUD) [Citation9,Citation11,Citation12] models. We performed the basic calculations and radiobiological modeling using our own dose-response fitting software which is based on the software package NPSOL (Nonlinear Programming, Systems Optimization Laboratory) [Citation13].
In this study the response probability P(D), in the RS model of the organ being uniformly irradiated to dose D, were calculated using the Probit model.
where D50 is the dose corresponding to a 50% complication probability after uniform irradiation of the reference volume and g50 is the normalized dose-response gradient. From the RS model, the s parameter was also estimated, which is the RS parameter that characterizes the organization of the organ. The parameters estimated from the Lyman model were the D50, the volume parameters n and m, which are inversely related to the slope of the dose-response curve or the normalized dose-response gradient γ50.
The maximum-likelihood method was used to estimate the parameters of the different models for each OAR. The Log Likelihood (LL) values were used to measure the goodness of fit and compare the information content of the different models. The 68% confidence intervals (CI) were also calculated for the model parameters. All statistical tests were performed at 5% significance level. To investigate the accuracy of the volume parameters the coefficient of variation [Citation14] and Pearson's correlation coefficient r were calculated. To compare survivors’ characteristics between survivors with and without the symptom we used Fisher's exact test for categorical variables and Wilcoxon-Mann-Whitney's rank sum test for continuous variables. To test whether there was a difference in mean and maximum dose received in each organ between patients with and without the symptom, a t-test was applied. The Wilcoxon test was also used to compare the cumulative DVHs. The Area under the Receiver Operating Characteristic Curve (AUC) was also calculated to identify which OAR dose (mean or maximum) was best correlated with having the studied symptom. For both the preliminary and main study multivariate analyses were performed using the Probit model and forward selection including mean and maximum doses, demographic, obstetrics, co-morbidities and treatment received. The variables were described in detail in one of our papers [Citation4].
Results
In the demographics and clinical characteristics of survivors with and without the symptom of ‘emptying of all stools into clothing without forewarning’ are illustrated. The tumor diagnoses of the patients were found to be associated with the symptom. Averaged total dose, as calculated from the treatment planning system, was 37.1 Gy (SD: 11.9) for endometrial cancer and for sarcomas 49.2 Gy (SD: 3.6). Ovarian cancer had an average total dose of 39.3 Gy (SD: 2.4), vulvar cancer 39.9 Gy (SD: 4.9) and cervical cancer 57.4 Gy (SD: 12.8). We observed that cervical cancer received the highest average total dose and also had the highest risk (6/15) of developing the symptom. A multivariate analysis has been performed and only the maximum dose to the anal sphincter was significant.
Table I. Demographic and clinical characteristics of long-term survivors with or without ‘emptying of all stools into clothing without forewarning'after pelvic radiation therapy at time of follow-up.
The treatment characteristics of the 77 cancer survivors included in the study are presented in . The table shows that survivors not having surgery (p = 0.0038) are more likely to develop the symptom. The survivors who had surgery received a lower average total dose (42.3 Gy, SD: 7.1) than survivors who had no surgery (66.2 Gy, SD: 2.5). The average total doses for survivors who had the symptom and for survivors not having the symptom were 54.2 Gy (SD: 11.9) and 43.2 Gy (SD: 8.9), respectively. illustrates that the difference between the mean doses among survivors with and without the symptom was significant for anal sphincter (p = 0.011), rectum (p = 0.0094), and sigmoid (p = 0.0069) but not for small intestines (p = 0.17). shows that the DVHs for the four OARs were significantly separated (p < 0.05) for doses 15–37 Gy and 41–67 Gy for anal-sphincter region, 44–69 Gy for rectum, 43–70 Gy for the sigmoid and 47–70 Gy for small intestines.
Table II. Treatment characteristics of long-term survivors with and without ‘emptying of all stools into clothing without forewarning’.
Table III. Mean and maximum absorbed doses for the four OARs and association tests (AUC, t-test p-value), comparing patients with and without the symptom of ‘emptying of all stools into clothing without forewarning’. Correlation coefficients (r) between mean and maximum absorbed doses.
Figure 1. Dose-volume histograms of the anal-sphincter region, the rectum, the sigmoid and the small intestines for survivors who developed the symptom (solid line) and survivors who did not develop the symptom (dashed-line).
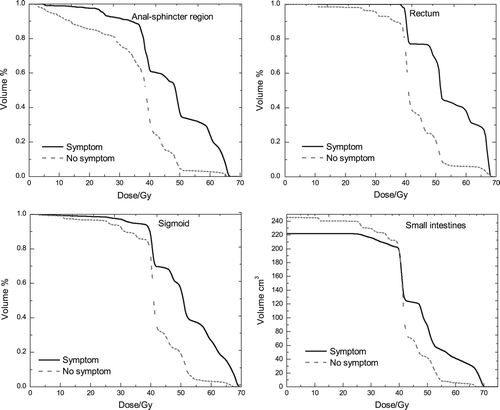
The estimates of the dose-response parameters using RS, Lyman and the gEUD model for anal-sphincter region, rectum, sigmoid and small intestines are presented in . The table also shows that of the four OARs, the anal sphincter and the sigmoid have the highest value of AUC (0.74). However, the sigmoid also has the highest γ50- value (1.60). The LL values () and the dose-response curves () indicate that the three studied models fit our data equally well especially for low and intermediate doses. In the corresponding dose-response curves are illustrated for the four OARs, using both the RS and the Lyman model, and the figure shows that the dose-response curves of the two models are very similar. The figure also shows that the sigmoid has the steepest curve and thus the highest dose-response relationship. According to , we found values for the volume parameters for the four studied OARs, but the anal-sphincter region, rectum and small intestines had very high s and a (s = 7.3, 10, 15.8 a = 2.3, 83.2, 119) values and thus almost no volume effect. Therefore the volume effect is shown only for the sigmoid in , using the RS model for a range of uniform doses. In , it may be seen that the range of mean doses of the survivors with the symptom is 37–60 Gy, while for the ones without the symptom 11–65 Gy. The figure also illustrates that the sigmoid behaves weakly in serial fashion.
Table IV. The maximum likelihood estimates of the dose-response parameters for the three OARs using RS, Lyman, gEUD model and the values for the AUC (area under the ROC curve).
Figure 2. Dose-response curves using the Relative Seriality and Lyman model, for the anal-sphincter region, rectum, sigmoid and small intestines.
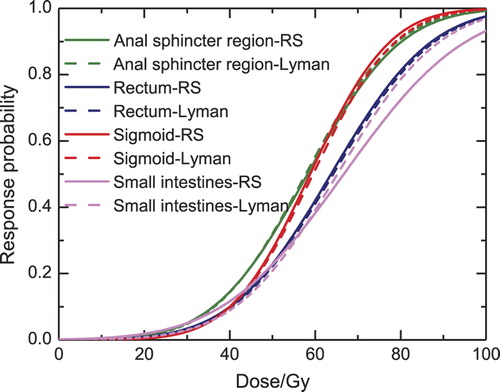
Figure 3. Volume effect using the Relative Seriality model for the sigmoid, assuming that the whole organ (100%) receives a uniform dose; two thirds (67%) of the organ receives a uniform dose and the remaining part receives 5% of this dose; and one third (33%) of the organ receives a uniform dose and the rest 5% of this dose. The light grey lines show the survivors with the symptom (responders) and the dark grey lines the survivors without the symptom (non-responders).
Discussion
In the present study the dose-response relationship for four OARs and the symptom ‘emptying of all stools into clothing without forewarning’ was investigated. Steep dose-response relationships were found for the four studied OARs and the highest γ50-value was for the sigmoid. However, in multivariate analyses only the maximum dose to the anal-sphincter region turned out to be statistically significant.
The volume parameters obtained for the anal-sphincter region (s = 7.3, n = 0.45), rectum (s = 10, n = 0.012), sigmoid (s = 1.32, n = 0.13), and small intestines (s = 15.8, n = 0.0084) for the three investigated models indicate a serial behavior of the organs for this endpoint. The relative seriality parameter s had a very large value for the anal-sphincter region, rectum and the small intestines, indicating that there was no difference using the maximum dose and the relative seriality model. That the maximum dose best describes the dose-response relationships for large s values was also pointed out in Adamus-Gorka et al. [Citation15]. The sigmoid was found to have a serial behavior but for the RS model the CI of the volume parameters (2e-08–10.3) are very wide and for the Lyman model (0.016–0.65) they are wide enough to prevent us from making any strong conclusion for the organ's volume effect, which adds a considerable uncertainty to the implication of the s value.
The DVHs () for the studied OARs were significantly separated for intermediate and high doses. The values of the coefficient of variation () are very small, mainly for the rectum but also for the sigmoid and the small intestines, which indicates that the absorbed doses for the individuals do not have large variations for these organs. The correlation coefficient r for these organs shows that the mean and maximum doses are almost linearly related, especially for the rectum. The values for the coefficient of variation and r for these organs imply that there will be a large uncertainty in the estimation of the volume effect. For the anal-sphincter region the coefficient of variation and r value, respectively, suggest that the doses vary for the individuals and the mean and maximum absorbed doses are not as clearly correlated linearly. Thus we expect to have better parameter estimation for the volume parameter due to the more heterogeneous dose distribution delivered.
Our results for the dose-volume response relations of RS, Lyman and gEUD models indicate that any one of the three studied models could be used, especially for low to intermediate doses, in order to predict the NTCPs of the symptom as a function of the dose to the anal sphincter, rectum, sigmoid and the small intestines. Consequently, the predicted response of all the four organs may be taken into account during a treatment planning in order to estimate the risk for developing the examined symptoms after RT.
To the best of our knowledge, there is no previous study estimating the dose response parameters of the sigmoid after RT for gynecological cancer. Fonteyne et al. [Citation16] reported late RT-induced lower intestinal toxicity (RILIT) for intensity- modulated radiotherapy of prostate cancer. They found a correlation between the sigmoid volume parameters and grade 1–2 RILIT and suggested that the sigmoid colon should be considered as an independent organ at risk. In our recent study we reported that there is a dose-effect relationship between mean absorbed doses > 50 Gy to the anal-sphincter region, the rectum, the sigmoid and the small intestines and the symptom of empting of all stools into clothing without forewarning [Citation4]. In the present study, as has been mentioned above, the DVHs are significantly separated for doses 15–37 Gy and 41–67 Gy for anal-sphincter region, 44–69 Gy for rectum, 43–70 Gy for the sigmoid and 47–70 Gy for small intestines.
For prostate cancer survivors, Mavroidis et al. [Citation17] reported an s = 0.37 for anal sphincter and fecal incontinence and Peeters et al. [Citation18] reported an n = 7.5 value for the anal wall and fecal incontinence. These results indicate a parallel behavior for the anal sphincter and for fecal incontinence unlike the serial behavior indicated in the present study. Al-Abany et al. [Citation19] also reported that for prostate cancer increasing the dose from 45–55 Gy to a large portion of the anal-sphincter region increases the risk of fecal leakage.
In addition to the effects from the RT absorbed doses, other factors like current smoking, heart failure, angina pectoris and cardiac infarction that are not considered to be confounding factors were also found to influence the development of the ‘emptying of all stools into clothing without forewarning’. Alsadius et al. [Citation20] reported that current smokers among prostate cancer survivors had an increased risk (prevalence ratio of 4.7) of developing the symptom of sudden ‘emptying of all stools into clothing without forewarning’. Our results do not indicate statistically significant difference between current smokers with and without the symptom. We also [Citation4] presented data for the total group of 519 gynecological cancer survivors indicating that heart failure, delivery with high birth weight and lactose and/or gluten intolerance are related to the symptom of ‘emptying of all stools into clothing without forewarning’. In the present study, survivors with heart failure have been excluded and no factor other than RT dose was found statistically significant.
One limitation of our study is the unaccounted organ motions, especially for the sigmoid, rectum and small intestines. In addition to this, there are possible errors in the patients’ set-up. The calculation of the dose-volumes was based on only a snapshot of pretreatment CT scanning. The distance of the CT scan and the low resolution introduce an uncertainty in the delineation of the organ. Additionally, there is uncertainty regarding small-intestines dose-volume data, since the whole organ was not delineated. To increase precision on anal-sphincter delineation magnetic resonance imaging (MRI) could have been used but during the treatment period (1991–2003), MRI was not available for organ delineation either at Karolinska University Hospital or at Sahlgrenska. Another possible source of uncertainty is the varying follow-up times in the patient material, but since we suspect that this symptom seldom heals, we believe this can be accepted.
In conclusion, we investigated the dose to the organs anal sphincter, rectum, sigmoid and the small intestines and the relation with the symptom ‘emptying of all stools into clothing without forewarning’ and calculated the corresponding dose- response relationships. Dose to the anal sphincter and mean dose to the sigmoid appear to be the parameters most closely related with the symptom. Steep dose-response relationships were found for all four OARs and according to the NTCP models applied, a serial behavior was found for this endpoint for all four OARs. Hence, in order to eliminate the risk of ‘emptying of all stools into clothing without forewarning’ as a radiation induced symptom in the future, delineation of the sigmoid as well as the anal-sphincter region, the rectum and small intestines may be considered.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dunberger G, Lind H, Steineck G, Waldenström AC, Nyberg T, al-Abany M, et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int J Gynecol Cancer 2010;20:449–60.
- Maeda Y, Hoyer M, Lundby L, Norton C. Faecal incontinence following radiotherapy for prostate cancer: A systematic review. Radiother Oncol 2011;98:145–53.
- Lind H, Waldenström AC, Dunberger G, al-Abany M, Alevronta E, Johansson KA, et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: A population-based cohort study. Br J Cancer 2011;105: 737–45.
- Lind H. Towards Prevention of Pelvic Radiation Disease in Gynecological Cancer Survivors. Stockholm: Karolinska Institutet; 2011[thesis].
- Adamus-Górka M. Improved dose response modeling for normal tissue damage and therapy optimization. Stockholm: Stockholm University & Karolinska Institutet; 2008[Thesis].
- Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. International journal of radiation oncology, biology, physics 2010;76(3 Suppl):S3–9.
- ICRU. Prescribing, recording, and reporting photon beam therapy. In: ICRU Report 50. Bethesda, MD: ICRU; 1993.
- Källman P, Ågren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992;62:249–62.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13–9.
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys 1989;16:1623–30.
- Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys 1997;24: 103–10.
- Niemierko A. A generalized concept of equivalent uniform dose (EUD). Med Phys 1999;26:1100[abstract].
- Gill PE, Murray W, Saunders MA, Wright MH. User’s guide for NPSOL 5.0 : A FORTRAN package for nonlinear programming. Technical Report SOL 86–6. Stanford, California: Systems Optimization Laboratory; 2001.
- Rosner B. Fundamentals of biostatistics, 7th ed. Boston, USA:Brooks/Cole; 2010.
- Adamus-Gorka M, Mavroidis P, Brahme A, Lind BK. The dose-response relation for rat spinal cord paralysis analyzed in terms of the effective size of the functional subunit. Phys Med Biol 2008;53:6533–47.
- Fonteyne V, De Neve W, Villeirs G, De Wagter C, De Meerleer G. Late radiotherapy-induced lower intestinal toxicity (RILIT) of intensity-modulated radiotherapy for prostate cancer: The need for adapting toxicity scales and the appearance of the sigmoid colon as co-responsible organ for lower intestinal toxicity. Radiother Oncol 2007;84: 156–63.
- Mavroidis P, al-Abany M, Helgason AR, Ågren Cronqvist AK, Wersä P, Lind H, et al. Dose-response relations for anal sphincter regarding fecal leakage and blood or phlegm in stools after radiotherapy for prostate cancer. Radiobiological study of 65 consecutive patients. Strahlenther Onkol 2005;181:293–306.
- Peeters ST, Hoogeman MS, Heemsbergen WD, Hart AA, Koper PC, Lebesque JV. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006;66:11–9.
- al-Abany M, Helgason AR, Cronqvist AK, Lind B, Mavroidis P, Wersä P, et al. Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys 2005;61:1035–44.
- Alsadius D, Hedelin M, Johansson KA, Pettersson N, Wilderang U, Lundstedt D, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol 2011;101:495–501.