Abstract
Background. The 7th TNM staging system for non-small cell lung cancer (NSCLC) developed by the International Association for the study of Lung Cancer (IASLC) has been applied in Sweden since the beginning of the year 2010. The aim of this retrospective study was to evaluate the prognostic role of the 7th TNM staging system in a surgical Swedish patient cohort with node-negative NSCLC. Material and methods. We collected data from stage I patients (pT1-2 pN0, 6th TNM system) who underwent surgery for NSCLC at Karolinska University Hospital from 1987 to 2002. Tumors were restaged according to the 7th TNM version. Cox multivariate survival analysis was implemented in order to determine the prognostic impact of pathological stage when classified according to either the 6th or the 7th TNM systems. Results. The patient population consisted of 452 subjects. Tumor size was ≤ 3 cm in 51% of cases. The predominant histology was adenocarcinoma (53%) and lobectomy was the most common surgical procedure (82% of patients). The five-year survival rate in patients with stage IA vs. IB (6th TNM) was 62% vs. 51%, respectively (log-rank p = 0.036). Corresponding figures for the 7th TNM system were 70% in stage IA-T1a, 51% in stage IA-T1b, 54% in stage IB, 51% in stage IIA and 35% in stage IIB (log-rank p = 0.002). On multivariate analysis, adjusted by age, gender, histology, kind of surgery, grade of differentiation and smoking status, pathological stage was an independent prognostic factor if classified according to the 7th TNM version (p = 0.001), but not if scored according to the 6th TNM edition (p = 0.090). Conclusion. The 7th TNM classification system is a more accurate predictor of prognosis in stage I operated patients than the old classification. The new system should be implemented even on retrospective cohorts especially when investigating the prognostic implication of the expression of molecular biomarkers.
Lung cancer is a major healthcare problem, being the most frequent cause of cancer-related deaths in both men and women worldwide [Citation1]. Similar figures are observed in Sweden, with a dramatic 3.6% increase per year in incidence in women in the last decade [Citation2].
Approximately 25–30% of cases with non-small cell lung cancer (NSCLC) are diagnosed with an early stage of disease and are amenable of curative treatment with surgical resection. However, in spite of diagnostic improvements, systematically lymph node dissection during lung cancer surgery and the addition of post-operative chemotherapy, an average of 35% and 55% of cases with stage I and II disease, respectively, do suffer tumor recurrence and die of lung cancer [Citation3]. In order to identify what patients are at higher risk of disease recurrence, robust and validated clinical and molecular prognostic factors should be implemented.
The tumor node metastasis (TNM) staging system is still the strongest clinical prognostic factor in NSCLC and plays a predominant role in the choice of treatment modality. The old 6th TNM staging version, based on data of approximately 5000 patients was published by C. F. Mountain in 1997 [Citation4].
In 1999 the International Association for the study of Lung Cancer (IASLC) started the collection of patient data in order to elaborate and propose to the International Union against Cancer (UICC) a more accurate staging system for the new TNM version. Changes proposed by IASLC have been fully accepted and constitute the current 7th TNM classification that is presently being implemented in the clinical setting [Citation5,Citation6].
Early stage node-negative tumors represent the majority of surgical cases. In this category the specific changes presented in the 7th TNM edition comprise a further sub-classification of stage IA into T1a and T1b, if the larger tumor diameter is up to or greater than 2 cm, respectively, and a move to stage IIA of cases with a diameter between 5 and 7 cm, and to stage IIB of tumors larger than 7 cm (). In addition, in this subgroup of patients, the use of post-operative chemotherapy is still controversial. In fact, results from a recent meta-analysis show that platinum-based adjuvant chemotherapy may be detrimental in stage IA [hazard ratio (HR) 1.19, 95% confidence interval (CI) 0.84–1.68, p = 0.33] and may improve five-year overall survival by 5% in stage IB cases (from 55% to 60%) [Citation7]. Hence, a need of an early identification of patients who would suffer tumor recurrence would be of high value. Moreover, when performing biomarker studies in this specific patient cohort, it is of great importance to correct biological findings with strong clinical prognostic factors, in order to avoid the risk of false positive results.
Table I. Comparison between 6th and 7th TNM systems for N0 tumors (modified from Rami-Porta et al. [Citation5].
The aim of the present retrospective study was thus to compare the 6th and 7th TNM classification systems in node-negative, surgically resected NSCLC cases, in order to define which of the two systems retains the strongest prognostic value in this particular patient population.
Material and methods
Subjects included in the present study where selected from the surgical lung cancer registry established in the year 1987 in the Stockholm-Gotland region, Sweden. Eligible cases were patients who received a curative and radical (R0) surgical resection for node-negative NSCLC and had a post-surgical follow-up of at least five years. Pre-operative staging was performed with chest x-ray only until 1993. Thereafter, CT-scan of the thorax and upper abdomen was introduced as routine imaging method for staging purposes. Patients with one of the following conditions were considered ineligible: 1) diagnosis of either neuroendocrine lung cancer or NSCLC with mixed neuroendocrine features [Citation8]; 2) two synchronous tumors; 3) pathological stage > I according to the 6th TNM staging edition; and 4) peri-operative chemotherapy. Tumors with mainly bronchoalveolar features were included because the staging classification was based on pathological specimens (pTNM) and not on clinical evaluations. In addition, formalin-fixed and paraffin-embedded tumor blocks had to be available from the County Council's biobank for pathological review of original diagnosis.
Follow-up data were provided by the Swedish Cancer registry. However, single patient files were also carefully reviewed in order to retrieve additional information not present in the registries such as smoking history, tumor localization, time-to- progression and metastasis localization. The post-operative follow-up consisted of clinical visit (history and physical) and chest x-ray approximately every three months for a total of five years.
Pathology reports describing the gross and microscopic examination of the surgical specimens containing the primary tumor and regional lymph nodes were collected. Cases were re-classified according to both the 6th and 7th TNM editions.
The study was approved by the Institutional Review Boards at Karolinska Institutet and at Stockholm´s County Council.
Statistics
Statistical analyses were performed using the JMP software 5.1.2 (SAS Institute, Cary, NC, USA).
Overall survival was calculated from date of surgical resection until death from any cause or last follow-up date. Assuming no disease recurrence after a follow-up of 10 years, patients surviving longer were censored at 120 months. Survival curves were calculated using the Kaplan-Meier method and compared by using the log-rank test. In order to determine the independent prognostic role of pathological stage scored according to either the 6th or the 7th TNM version, multivariate analysis by using the Cox proportional hazard method was conducted.
A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total number of 452 patients were included in this study. Subjects received curative surgery at the Department of Cardiothoracic Surgery and Anesthesiology, Karolinska University Hospital, Stockholm, Sweden, between March 1987 and December 2002. Patient characteristics are listed in . Median age was 68 years, and 40% of patients were elderly (aged > 70 years). The histological type was squamous-cell carcinoma in 32% of cases. Among the 240 adenocarcinomas, 72 tumors had also bronchioloalveolar features. All surgical procedures were performed with open thoracotomy and consisted of lobectomy (82%), bilobectomy (7%) or pneumonectomy (10%). The N0 condition was pathologically confirmed in all patients. Unfortunately, it was not possible to determine for each case how many lymph nodes were actually removed, due to inconsistent registration of this information in the pathology as well as in the the surgery reports. Mediastinal lymph node sampling was done randomly based on the macroscopic examination of the mediastinum during the operation for lung resection through the same thoracic incision.
Table II. Patient characteristics.
Changes in stage classification according to the 6th and the 7th versions
Approximately half of the cases were in either stage IA or IB according to the 6th TNM edition. Patients were classified according to both TNM systems (). Of tumors with a diameter ≤ 3 cm, 52% had a size of ≤ 2 cm and moved to T1a (7th TNM), and 48% had a size > 2 cm and moved to T1b (7th TNM). In terms of stage IB (6th TNM), the majority of cases were still in this staging class even according to the 7th TNM classification because of tumor size ≤ 5 cm. However, approximately 35% of cases moved to either stage IIA or IIB ().
Table III. Classification of 452 node-negative NSCLC patients according to the 6th and 7th TNM systems.
Survival analysis and risk of metastasis
At the time of data analysis 299 subjects had died (66%). Minimum follow-up time in 153 living patients was 63 months (median 120 months).
Univariate survival analyses by tumor size are presented in , where Kaplan-Meier curves according to the 6th TNM classification and the further sub-grouping according to the 7th system are depicted together. In terms of stage IA tumors, five-year overall survival rates were 70% vs. 51% in stage IA–T1a and IA–T1b, respectively (log-rank p-value = 0.001). The five-year survival rate was 51% in patients with a tumor size > 3 cm. Further sub-grouping according to the 7th TNM edition resulted in five-year overall survival rates of 54%, 51% and 35% in stage IB, IIA and IIB, respectively (log-rank p-value = 0.150). depicts five-year survival rates and univariate overall survival analyses by patient characteristics.
Figure 1. Kaplan-Meier curves of overall survival comparing the 6th TNM system (stage IA in A; stage IB in B) with the patient sub-grouping resulting from the 7th TNM classification.
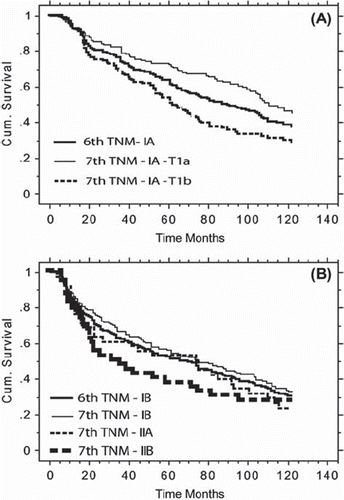
Table IV. Univariate survival analysis.
Multivariate survival analyses were adjusted by gender, age (≤ 70 years vs. > 70 years), histology (squamous vs. non-squamous tumors), grade of differentiation (low vs. moderate vs. high), smoking status (never vs. former vs. current smokers) and kind of surgery (lobectomy vs. pulmectomy-bilobectomy). Pathological stage was included in each model and scored according to either the 6th or the 7th TNM classifications. In terms of overall survival, stage was not an independent prognostic factor if scored according to the 6th edition. HR of death for stage IA (vs. IB) was 0.90 (95% CI 0.80–1.02), p-value = 0.090. Conversely, it was indeed an independent prognosticator when classified according to the 7th TNM version (p-value = 0.001). Using stage IIB as reference, HR (95% CI) was 1.10 (95% CI 0.79–1.50) for stage IIA, 0.96 (0.78–1.17) for stage IB, 1.02 (0.81–1.27) for stage IA–T1b and 0.68 (0.53–0.87) for stage IA–T1a.
Discussion
In the present study we performed a retrospective analysis in order to determine whether the newly introduced 7th TNM classification system for early stage node-negative NSCLC is a more accurate prognosticator of overall and disease specific survival than the previous 6th TNM system. Adjusted multivariate survival analysis showed that the 7th TNM system was indeed a more accurate classification than the 6th TNM to predict the overall survival of patients in this cohort. This was likely an effect of the further separation of stage IA tumors into two cohorts, which resulted into the identification of tumors with a size < 2 cm as a clear group with better outcome, with a five-year survival rate of 71%, compared to the approximately 50% observed in stage IA–T1b until IIA. This newly introduced subclassification of T1 tumors seems to retain a strong prognostic potential, being statistically significant on multivariate overall survival analysis.
Our study encompasses only early stage pN0 cases treated with curative surgical resection, without the addition of adjuvant or neoadjuvant treatments. This choice was based on the following considerations. With the introduction of well-established technologies to refine diagnosis, such as the pre-operative use of PET-CT, the majority of currently operated NSCLC cases are pN0 tumors [Citation9,Citation10], since it is praxis to treat patients with positive N2 disease with chemoradiation although surgical resection could be technically feasible. As a consequence, biomarkers research requiring archival surgical material for extensive analyses will be mainly performed on node-negative cases. In addition, the use of adjuvant chemotherapy in this group of patients is still debated [Citation7,Citation11]. Hence, a detailed study on clinical prognostic factors in this group of patients is clinically relevant. The main advantages of our study are the long patient follow-up and the accuracy of clinical data included in multivariate analysis, e.g. smoking status. The major limitations are the absence of further classification of visceral pleural invasion (VPI) and of lymphovascular invasion (LVI), although the prognostic role of these factors is still debated [Citation12–14] and the lack of detailed information about mediastinal lymph node dissection, whether performed with sampling or systematic. As far as this latter issue is concerned, according to Lardinois et al. [Citation15] systematic lymph node dissection is generally considered the procedure of choice even in pre-operative cN0 cases. However, a recent randomized trial from Darling et al., which allocated 1111 T1–2, N0–1 patients to either sampling or systematic dissection, showed no difference in overall survival and local recurrence rate between the two procedures after a median follow-up of 6.5 years [Citation16]. The staging accuracy is important, then it can contribute to patient selection for adjuvant chemotherapy and prediction of prognosis but when it comes to the question of a possible benefit of systematic lymph node dissection in stage I patients, it is still controversial.
Since the results of the IASLC staging project were published and a proposal for a 7th TNM version for staging NSCLC was released, a number of papers have investigated the applicability of the new system into retrospective series [Citation3,Citation14,Citation17–21].
Each study explores different aspects of the problem, making difficult direct comparisons, especially because of the heterogeneity of the patient population in terms of, e.g. histology and nodal involvement. However, very few papers perform a direct head-to-head comparison between the 6th and 7th TNM versions in terms of prognostic implications, as we instead do in the present report [Citation14,Citation20].
One of the major advances of the 7th TNM system is the importance given to the further subclassification of tumors by size, especially with regard to surgical cases. Nonetheless, previous experiences concerning the prognostic role of tumor size were conflicting. Whereas Li et al. [Citation14] found that the tumor size was a significant independent prognostic factor in stage I NSCLC and Veeramachaneni et al. showed that in Stage IA the increase of tumor diameter by 1 cm increased the risk of nodal metastases by a factor of 3.5 with significant decrement in survival [Citation22], an earlier study from Duke University found that in a similar patient cohort there was no statistic relationship between tumor size and survival [Citation23]. Similarly, Takeda et al. reported that in node negative NSCLC patients there was no significant prognostic difference among tumors up to 5 cm [Citation24]. Conversely, Ruffini et al. recognized the crucial role of tumor size as a prognostic indicator but also noticed the importance of the way the tumor size is measured and the fixation process after resection [Citation21]. Whenever possible it should be measured on the fresh specimens and calculated on the longest diameter, otherwise it could theoretically falsely downstage about 10% of cases. In addition, another interesting consideration in the study from Ruffini et al. [Citation21], is that the IASLC does not further investigate or elucidates the ‘non-size-based’ descriptors of T2 tumors, i.e. the airway location of the tumor and its local invasion, which were instead mentioned and recognized in the 6th TNM version [Citation25].
Further strengthening the role of tumor dimension, a Japanese study has shown that after the spread implementation of the lung cancer mass screening system (started 1987 with plain chest radiograph plus sputum cytology for unemployed residents), the detection of early stage lung tumors with a size of 2 cm or less has increased, leading to a five-year survival rate improvement from 47.8% in 1989 to 62% in 1999 [Citation26].
In addition to tumor size, other pathological factors such as the lymphovascular invasion and the visceral pleural involvement have been described as valuable prognostic factors for the five-year survival rate [Citation14,Citation19,Citation27]. Interestingly, Yano et al. report a statistically significant difference in the incidence of lymphatic permeation in node-negative T1a and T1b (4.5% vs. 12%, respectively) [Citation19]. However, to what extent this information may also be relevant in order to predict the benefit of adjuvant chemotherapy is presently not clear.
In conclusion, the results of our retrospective study confirm that the 7th TNM classification system is a more precise predictor of prognosis in stage I operated patients than the 6th TNM version. The newly introduced subclassification of T1 tumors seems to retain a strong prognostic potential, being statistically significant on multivariate overall survival analysis. The 7th TNM system should furthermore be implemented even on retrospective cohorts especially when investigating the prognostic implication of the expression of molecular biomarkers.
Acknowledgements
Supported in part by grants from Karolinska Institutet (KID funding), Stockholm County Council, Swedish Cancer Society and Stockholm Cancer Society. Special thanks to the Kleberg Foundation whose grants provided the time to accomplish this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Globocan 2008. Available from: http://www.globocan.iarc.fr [cited 25 June 2012].
- The national lung cancer registry in Sweden. Available from: http://www.roc.se/lungca.asp [cited 25 June 2012].
- Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, et al. The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694–705.
- Mountain CF. Revisions in the international system for staging lung cancer. CHEST 1997;111:1710–7.
- Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593–602.
- Goldstraw P, Crowley JJ, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706–142.
- Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. NSCLC Meta-analyses Collaborative Group. Lancet 2010;375:1267–77.
- Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch Pathol Lab Med 2010;134:1628–38.
- Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 2009;361:32–9.
- Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predicts recurrence in stage I lung cancers. J Thorac Oncol 2011;6:43–7.
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9.
- Manac´h D, Riquet M, Medioni J, Le Pimpéc-Barthes F, Dujon A, Danel C. Visceral pleura invasion by non-small cell lung cancer: An underrated bad prognostic factor. Ann Thorac Surg 2001;71:1088–93.
- Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, Morishita Y, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:160–5.
- Li Z, Yu Y, Lu J, Luo Q, Wu C, Liao M, et al. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J Thorac Oncol 2009; 4:702–9.
- Lardinois D, De Leyn P, Van Schil P, Rami Porta R, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787–92.
- Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662–70.
- Kameyama K, Takahashi M, Ohata K, Igai H, Yamashina A, Matsuoka T, et al. Evaluation of the new TNM staging system proposed by the International Association for the Study of Lung Cancer at a single Institution. J Thorac Cardiovasc Surg 2009;137:1180–4.
- Kassis ES, Vaporciyan AA, Swisher SG, Correa AM, Bekele BN, Erasmus JJ, et al. Application of the revised lung cancer staging system (IASLC Staging Project) to a cancer center population. J Thorac Cardiovasc Surg 2009;138:412–8.
- Yano T. Morodomi Y, Ito K, Yoshida T, Haro A, Shoji F, et al. Verification of the newly proposed T category (seventh edition of the tumor, node, and metastasis classification from a clinicopathological viewpoint in non-small cell lung cancer-special reference to tumor size. J Thorac Oncol 2010;5:45–8.
- Strand TE, Rostad H, Wentzel-Larsen T, von Plassen C. A population-based evaluation of the seventh edition of the TNM system for lung cancer. Eur Respir J 2010;36:401–7.
- Ruffini E, Filosso PL, Bruna MC, Coni F, Cristofori RC, Mossetti C, et al. Recommended changes for T and N descriptors proposed by the International Association for the Study of Lung Cancer – Lung Cancer Staging Project: A validation study from a single-centre experience. Eur J Cardiothorac Surg 2009;36:1037–44.
- Veeramachaneni NK, Battafarano RJ, Meyers BF, Bell Zoole J, Patterson GA. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: A negative impact on survival. Eur J Cardiothorac Surg 2007;33:466–9.
- Patz EF, Rossi S, Harpole DH, Herndon JE, Goodman PC. Correlation of tumor size and survival in patients with stage IA non-small cell lung cancer. Chest 2000;117:1568–71.
- Takeda S, Fukai S, Komatsu H, Memoto E, Nakamura K, Murakami M. Impact of large tumor size on survival after resection of pathologically node negative (pN0) non-small cell lung cancer. Ann Thorac Surg 2005;79:1142–6.
- Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic significance of the non-sized-based AJCC T2 descriptors: Visceral pleura invasion, hilar atelectasis or obstructive pneumonitis in stage IB, non-small cell lung cancer is dependent on tumor size. Chest 2008;133:662–9.
- Koike T, Yamato Y, Asamura H, Tsuchiya R, Sohara Y, Egushi K, et al. Improvements in surgical results for lung cancer from 1989 to 1999 in Japan. J Thorac Oncol 2009;4:1364–9.
- Ruffini E, Asioli S, Filosso PL, Buffoni L, Bruna MC, Mossetti C, et al. Significance of the presence of microscopic vascular invasion after complete resection of stage I–II pT1. T2N0 non-small cell lung cancer and its relation with T-size categories. J Thorac Oncol 2011;6:319–26.
