Abstract
Purpose. To determine if high-risk prostate cancer responds differently to hypofractionation. Material and methods. One hundred and fifty-seven men with NCCN high-risk (T3, PSA > 20, or Gleason ≥ 8) clinically localized prostate cancer treated between 1998 and 2010 met the inclusion criteria for the analysis. Eighty-two were treated with conventional WPRT with a conventionally fractionated sequential boost to the prostate (cRT), with the prostate receiving 75–77 Gy in 1.8–2.0 Gy fractions. Seventy-five were treated with pelvic IMRT with a hypofractionated simultaneous boost to the prostate (hRT), with the prostate receiving 70 Gy in 2.5 Gy fractions. The dose to the pelvic lymph nodes was 45 Gy in the cRT group and 50.4 Gy in the hRT group, both at 1.8 Gy per fraction. Ninety-two percent received neoadjuvant hormonal ablation therapy, typically beginning two months prior to the start of RT. Results. Median follow-up was 6.5 years for men receiving cRT and 3.7 years for those receiving hRT. The actuarial rate of biochemical control at four years was 88% for cRT and 94% for hRT (p = 0.82). The rates of early rectal and urinary grade ≥ 2 toxicities were 35% (29 of 82) and 49% (40 of 82) for the cRT group and 36% (27 of 75) and 44% (33 of 75) for the hRT group. The actuarial rate of late grade ≥ 2 rectal toxicity at four years was 25% for the cRT group and 13% for the hRT group (p = 0.037). The rate of late grade 3 rectal complications was 4% (3 of 82) for patients receiving cRT and 1% (1 of 75) for patients receiving hRT. Conclusion. Initial follow-up indicates equivalent biochemical control between regimens. Patients receiving hRT experienced fewer late rectal complications.
High-risk prostate cancer remains difficult to control despite the improvements of modern treatment regimens. The M.D. Anderson dose-escalation trial revealed improved biochemical control in patients with initial PSA > 10 ng/mL [Citation1]. More recently, Pahlajani et al. have published similar results for patients with Gleason scores ≥ 8 [Citation2]. To date, the response of high-risk prostate cancer to hypofractionation has not been directly addressed.
Low estimates of the alpha-beta ratio of prostate cancer, such as 1.8 Gy, as described by Leborgne et al., help to provide radiobiologic justification for hypofractionation [Citation3]. However, such estimates tend to be based on poorly matched groups that do not take into account pretreatment characteristics [Citation4]. By definition, tumors with higher Gleason scores exhibit increased cellular atypia and therefore may respond differently to increasing fraction size. To date, there has been no published estimate of the alpha-beta ratio of specifically high-risk prostate cancer.
Treatment of the pelvic lymph nodes also remains a controversial technique. Though the results of RTOG 9413 initially appeared to support nodal irradiation, long-term results were inconclusive [Citation5]. There have been two prospective phase I studies investigating hypofractionated treatment to the prostate with simultaneous nodal irradiation, with biochemical control rates of 81.2% at three years [Citation6] and 90.5% at four years [Citation7] for high-risk patients. Pelvic lymph node irradiation in the setting of hypofractionated boost to the prostate was also incorporated into the Fox Chase phase III hypofractionation trial as a result of the initial RTOG 9413 results. However, the long-term results of this trial have only been presented in abstract form without a comparison of patients who did and did not receive nodal irradiation [Citation8].
Our aim in this analysis was to compare two different fractionation schemes of prostate radiotherapy in the setting of conventionally fractionated pelvic nodal irradiation for high-risk prostate cancer. We predicted that outcomes for patients with high-risk disease treated with the hypofractionated regimen would be non-inferior to those of patients treated with the conventional regimen.
Methods
Inclusion criteria
The records of every patient receiving radiation treatment for localized prostate cancer at the University of Alabama at Birmingham (UAB) since 1998 were reviewed. All patients meeting the following requirements were included in the analysis: biopsy-proven National Comprehensive Cancer Network (NCCN) high-risk prostate cancer (T3, PSA > 20, or Gleason ≥ 8), clinically localized disease, no previous treatment other than hormonal ablation, dose-escalated external beam radiation with treatment to the pelvic lymph nodes, and follow-up ≥ 1 year. Data collection was approved by the University of Alabama at Birmingham Institutional Review Board.
Staging
The staging process was nearly identical for all patients included in this analysis. For all patients, tissue specimens were reviewed by the UAB Department of Pathology; only Gleason scores reported by the internal review were taken into account for this analysis. The highest pretreatment PSA was utilized regardless of where this testing occurred. Digital rectal examination was always performed during the initial consultation, however, additional imaging modalities were also utilized in some cases to aid in determining the T stage at the discretion of the practitioner. For patients with T3 disease, the manner by which this was determined is included in . Additionally, all patients were evaluated for the presence of metastatic disease by both Tc-99m bone scan and CT of the abdomen and pelvis.
Table I. Pretreatment characteristics.
Treatment planning and delivery
All patients underwent CT simulation, which was performed in the supine position with standard immobilization. Patients were ensured to have a full bladder and empty rectum at the time of simulation. The rectum was contoured as a solid organ from the level of the ischial tuberosities inferiorly, to the rectosigmoid junction superiorly. Other avoidance structures contoured included the entire bladder (with contents) and femoral heads and greater trochanters. The prostate clinical target volume (CTV1) was defined as the prostate along with any visible areas of tumor extension and the seminal vesicle CTV (CTV2) was defined as the entirety of the seminal vesicles.
Eighty-two patients received conventionally fractionated treatment between 1998 and 2007. The initial phase of treatment consisted of 45 Gy delivered in 1.8 Gy fractions to the pelvis via a four-field box technique, with the upper field border set at the superior level of the sacroiliac joints and inferior border 2 cm inferior to the prostate. The field was then reduced to a planned treatment volume (PTV) consisting of the prostate and seminal vesicles (CTV1 and CTV2) plus a 1 cm margin. This volume was prescribed a 10 Gy boost in 2.0 Gy fractions delivered via and 3D conformal therapy (3D-CRT). The treatment field was then further reduced to a PTV consisting of the CTV1 with a 0.5 cm margin in all directions. This volume was prescribed a 20–22 Gy boost in 2.0 Gy fractions delivered via 3D-CRT in 41 (50%) patients and intensity-modulated radiotherapy (IMRT) in 41 (50%) patients. The final boost to the prostate was 20 Gy in 32 (39%) patients and 22 Gy in 50 (61%) patients. For patients receiving the final boost via IMRT, ultrasound guidance via a B-mode acquisition and targeting system (BAT) or megavoltage CT was utilized to verify the positioning of the prostate. Conventionally fractionated treatment was delivered by Varian linear accelerators with the exception of a small minority of patients whose final boost was delivered by a TomoTherapy machine.
Seventy-five patients received hypofractionated treatment between 2005 and 2010. For IMRT planning, CTV1 and CTV2 were defined as in the conventionally fractionated regimen. The planning target volume for the prostate (PTV1) consisted of the CTV1 as well as a 7 mm extension in all directions, except posteriorly where the extension was 4–5 mm. The seminal vesicle PTV (PTV2) consisted of the CTV2 plus a 7 mm extension in all directions, except posteriorly where the extension was 4 mm. The nodal clinical target volume (CTV3) was generated by contouring a 7 mm extension around the internal iliac, external iliac, and common iliac vessels to the level of mid-S1 in order to approximate a beam aperture at the level of the L5-S1 junction. The nodal PTV (PTV3) was then generated by extending the CTV3 7 mm in the lateral directions and 9 mm anteriorly and posteriorly. The treatment prescription consisted of 50.4 Gy to the PTV3 with simultaneous 56 Gy to the PTV2 and 70 Gy to the PTV1 all delivered simultaneously over 28 fractions of 1.8 Gy, 2.0 Gy, and 2.5 Gy, respectively. Hypofractionated treatment was delivered by a TomoTherapy machine or Varian 2100 linear accelerator. Daily image guidance was performed by megavoltage CT (TomoTherapy) or cone-beam kilovoltage CT (Varian) prior to each fraction. For CT-based image guidance, the alignment was performed to the prostate-rectal interface via a rigid translation of the plan; a small minority of patients also underwent fiducial seed implantation to facilitate image-guidance.
Rectal constraints limited the volume receiving 60 Gy (V60) to the lesser of 10 cm3 or 10% of the total rectal volume. No predefined bladder constraints were utilized for patients receiving conventionally fractionated treatment, but high doses were minimized on an individualized basis. For patients receiving hypofractionated IMRT, bladder constraints limited the V40 to 50% of the total bladder volume and the V65 to 25% of the total bladder volume.
IMRT plans were generated using inverse planning using TomoTherapy Planned Adaptive or Varian Eclipse software; 3D-CRT plans were generated by Varian Eclipse software. TomoTherapy plans were required to deliver the whole of the prescription dose to a minimum of 95% of the PTV. Eclipse plans were required to deliver 95% of the prescription dose to the whole of the PTV.
Androgen deprivation
Androgen deprivation therapy was typically begun six to eight weeks prior to the delivery of the first fraction. The choice of agent varied slightly by practitioner, but consisted of an anti-androgen (typically bicalutamide) in combination with an LHRH agonist. The total length of androgen deprivation ranged from six months to three years.
Toxicity evaluation and follow-up
Patients were seen weekly during the course of treatment, and toxicities, if any, were recorded and treated. Return visits were scheduled every three to four months for the first two years following RT, and every six months thereafter. Rectal and urinary toxicity was graded with the Common Terminology Criteria for Adverse Events 4.0 guidelines [Citation9]. Only the highest grade early and late toxicities for each patient were taken into account for the analysis. Late toxicity was defined as new symptoms appearing greater than three months from the completion of RT. Early toxicities continuing for > 3 months from the completion of RT were recorded as early toxicity. In general, patients with rectal bleeding were initially treated with steroid suppositories, and those refractory to therapy were referred for endoscopic laser procedure. Patients with diarrhea more than twice per week were prescribed anti-diarrheal medications. PSA levels were repeated typically every six months and at least every 12 months following the completion of RT. Biochemical failure was defined using the RTOG-ASTRO Phoenix definition as a rise of 2.0 mg/mL above the nadir regardless of hormone therapy status [Citation10].
Statistical methods
Statistical analysis of the data was performed using IBM SPSS Statistics 20.2 software. The actuarial rates of biochemical freedom from progression and freedom from toxicity were calculated using the Kaplan-Meier method. Log-rank testing was used to determine statistically significant differences between groups. Biochemical freedom from progression was calculated from the beginning of androgen deprivation therapy (or beginning of RT if androgen deprivation was declined) and freedom from toxicity was calculated from the beginning of RT. Patients were censored from the analysis at the time of the most recent follow-up.
Means were compared by the independent samples t-test and frequencies were compared by the Pearson's χ2 method. Data collection was approved by the University of Alabama at Birmingham Institutional Review Board.
Results
Pretreatment characteristics
One hundred and fifty-eight patients met the inclusion criteria for the analysis. Eighty-two were treated with conventionally fractionated pelvic RT followed by conventionally fractionated sequential boost (cRT) between 1998 and 2007; 75 were treated with hypofractionated image-guided pelvic IMRT with a hypofractionated simultaneous integrated boost to the prostate (hRT). Ninety-seven patients had Gleason score ≥ 8, 69 had initial PSA > 20, and 27 had T3 disease by digital rectal exam or MRI. Ninety-two percent of patients received neoadjuvant hormonal ablation. A comprehensive list of pretreatment characteristics is presented in ; aside from slightly more cases of Gleason score ≤ 6 in the cRT group, there were no statistically significant differences between the two treatment groups.
Biochemical control
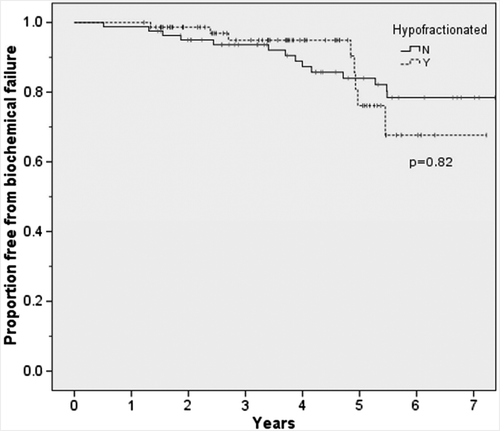
Median follow-up was 6.5 years for men receiving cRT and 3.7 years for those receiving hRT. The actuarial rate of biochemical control at four years was 88% for cRT and 94% for hRT (p = 0.82). The Kaplan-Meier plots of biochemical freedom from progression are presented in .
Acute toxicity
Of the patients receiving cRT, 35% (29 of 82) experienced early grade ≥ 2 rectal complications and 49% (40 of 82) experienced early grade ≥ 2 urinary complications. The hRT regimen resulted in 36% (27 of 75) patients with early rectal complications and 44% (33 of 75) with early urinary complications. There were no statistically significant differences between the groups concerning early toxicity with p = 0.207 for early rectal complications and p = 0.206 for early urinary complications.
Late toxicity
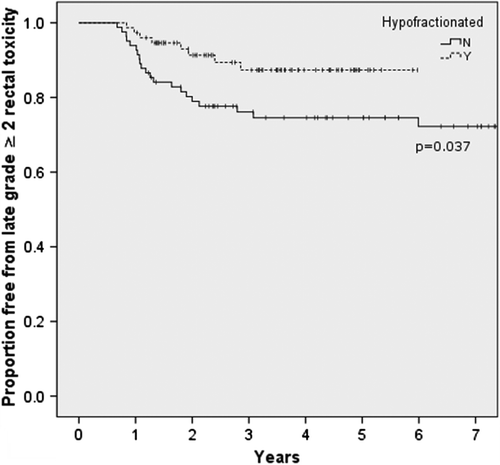
The actuarial rate of late grade ≥ 2 rectal toxicity at four years was 25% for the cRT group and 13% for the hRT group (p = 0.037). The Kaplan-Meier estimates of freedom from late rectal toxicity are shown in . The majority of events were grade 2. Three patients who received conventional treatment experienced grade 3 complications as did one patient who received hRT. Of the grade 3 complications, all were related to prolonged rectal bleeding: two patients required an endoscopic coagulation procedure and two patients required blood transfusion. There were no grade 4 rectal complications in either group.
At four years, the actuarial rate of grade ≥ 3 urinary complications was 3% for patients receiving cRT, and 6% for patients receiving hRT (p = 0.52). One patient experienced a grade 4 urinary event consisting of a urethral stricture refractory to multiple dilatation procedures and eventually requiring surgical urethrotomy. Of the grade 3 events, two patients experienced urethral strictures which responded to urethral dilatation and four patients experienced transient gross hematuria attributed to radiation cystitis. A description of all late toxicity events is presented in .
Table II. Description of late toxicity events.
Discussion
To date there has been no prospective study published comparing conventional versus hypofractionated whole-pelvic radiation for the treatment of high-risk prostate cancer. We performed this retrospective study in order to compare the efficacy and toxicity of this controversial mode of therapy. We saw no statistically significant difference between the hypofractionated and conventionally fractionated groups concerning biochemical control and early toxicity. Conventional fractionation was associated with increased late toxicity (25% vs. 12% in the hypofractionated group at four years).
The most recent large multi-institutional prospective trials of conventionally fractionated whole-pelvic RT include RTOG 94-13 and GETUG-01. One arm of the RTOG 94-13 trial utilized whole-pelvic radiation combined with neoadjuvant hormonal ablation therapy to treat patients with at least a 15% risk of lymph node involvement as calculated by the Roach formula [Citation5]. The pelvis was prescribed 50.4 Gy followed by a sequential 19.8 Gy boost to the prostate, all delivered conventionally in 1.8 Gy fractions. Patients in this arm also underwent a four month course of androgen deprivation therapy beginning two months prior to the start of RT. An in-depth secondary analysis investigating the effect of the size of the pelvic fields reported 57.4% of patients treated in this arm to be disease-free at four years by the protocol definition. The rates of late grade ≥ 3 GU and GI toxicities at five years were 3.0% and 4.3%, respectively [Citation11]. The GETUG-01 study also contained an arm utilizing whole-pelvic radiation. A variety of dose schedules were allowed in this study, with pelvic doses ranging from 45 Gy to 46.8 Gy and total prostate doses ranging from 66 Gy to 72 Gy. Fraction size ranged from 1.8 Gy to 2.25 Gy and delivered conventionally or by 3D-CRT. Patients with high-risk disease also received a four to eight month course of neoadjuvant hormonal therapy delivered neoadjuvantly and concurrently. This treatment resulted in 59.8% of patients with high-risk disease to be disease-free at five years [Citation12]. Overall, 37.7% of patients developed late grade ≥ 2 GU toxicity and 31.7% developed late grade ≥ 2 lower GI toxicity. In comparison, the observed rates of late toxicity in our study are similar with estimates of 25% for late grade ≥ 2 GI events and 3% for late grade ≥ 3 GU events at four years. However, biochemical control rate for patients treated in our study compares favorably to the aforementioned trials with 88% of patients disease-free at four years, which may be attributed to the higher prostate dose 75 Gy to 77 Gy used to treat our patients. With the use of newer technology, it is now possible to achieve these dose levels without an incremental increase in morbidity.
Though a higher RT dose to the prostate is known to reduce rates of failure in patients with high risk prostate cancer few published studies have utilized doses higher than 75 Gy to treat the prostate [13,14 ]. In a retrospective study analysis comparing prostate-only versus whole-pelvic RT by Aizer et al., patients with high-risk disease were treated to a median prostate dose of 75.6 Gy using conventional fractionation [Citation15]. Early and late grade ≥ 2 rectal complications were 33% and 5.9%, respectively. In comparison, 36% patients in our study developed acute rectal complications. Our observed rate of late rectal toxicity for this group was substantially higher at 25%; however, rates of late rectal complications as high as 46.8% have been reported as a result of conventionally-fractionated WPRT [Citation16].
Hypofractionated radiotherapy has gained popularity over the years thanks largely to the feasibility of delivering highly-precise radiotherapy and a better understanding of tumor radiobiology. At our institution, the shift towards utilizing a hypofractionated regimen, as opposed to conventional fractionation, was based on promising retrospective data [Citation17], the acquisition of a TomoTherapy unit with daily image guidance capability and alpha/beta estimates of prostate cancer. The decision was reinforced by the early results of the Fox Chase trial [Citation1] and positive feedback from our patient population with respect to the shortened overall treatment time.
In a phase I study evaluating the feasibility of delivering 56 Gy to the pelvic lymphatics in high-risk patients, Adkinson et al. treated the pelvic lymphatics using IMRT to 56 Gy at 2.0 Gy per fraction with a simultaneous boost technique to 70 Gy in 28 fractions to the prostate, achieving biochemical control in 81.2% patients at three years. The rates of clinically significant early rectal and urinary toxicities were 32% and 37%, respectively. An 8% incidence of grade 2 late rectal and 2% incidence if grade 3 late urinary toxicity were reported [Citation6]. A slightly different fractionation scheme was utilized to treat high-risk patients by Quon et al. who prescribed 45 Gy to the pelvis in 25 fractions with a simultaneous boost to the prostate to a total of 67.5 Gy. The overall freedom from biochemical failure rate at four years was 90.5%, with early GI and GU toxicity rates of 36.3% and 43%, respectively. The crude rate of late rectal events was 7% [Citation7]. A retrospective analysis by McCammon et al. using dosing regimen identical to our study group, but without daily image guidance, showed 73% biochemical control at 45.7 months, with rectal and urinary toxicities closely corresponding to the results of our study [Citation18,Citation19]. The results of relevant studies are summarized in .
Table III. Summary of selected publications.
We saw reduced incidence of toxicity in the hypofractionated group despite a somewhat larger treatment volume (true pelvis vs. whole pelvis). If the alpha-beta ratio of prostate cancer is truly lower than surrounding rectal tissue, that alone would explain the lower late complication rate in the hypofractionated arm. However, it is very likely that treatment technique could also have impacted the outcome, since patients in the hypofractionated arm were entirely treated using IMRT while conventional regimen was delivered using either sequential 3D-CRT or IMRT boost. Additionally the group receiving hypofractionated therapy to the prostate were treated with the aid of daily cone-beam or megavoltage CT guidance for the entire length of treatment, whereas for patients receiving conventionally fractionated therapy image-guidance was only performed during the prostate boost. While the exact cause of the apparent reduced toxicity is difficult to assess, the toxicity rates reported in this study supports the use of hypofractionated RT with judicious treatment planning and IMRT to reduce toxicity rates.
The alpha-beta ratio of prostate cancer remains a point of controversy. From our experience, hypofractionated prostate RT with simultaneous pelvic nodal treatment appears to perform at least as well as conventionally fractionated WPRT in terms of biochemical control for high-risk prostate cancer. Whether or not this is a result of the improved treatment planning and delivery methods or to the intrinsic sensitivity of the tumor tissue to fraction size cannot be determined without prospective data. Long-term results from the Fox Chase trial utilizing hypofractionated versus conventionally fractionated prostate radiotherapy with simultaneous elective lymph node irradiation for high-risk prostate cancer will provide better information regarding the use of hypofractionation for the treatment of high-risk patients [Citation1,Citation8].
Though our study has shown both safety and efficacy with the use of a hypofractionated schedule for this patient group, it is important to note the limitations of this study. First, this study is limited by its retrospective nature and the different treatment techniques we have used over the span of the study period. However, well-defined inclusion criteria help to minimize bias, and the pretreatment characteristics of the two groups are well-matched, with the exception of more instances of Gleason ≤ 6 pathology in the cRT group. While we recognize this difference, we feel that the effect on the analysis is likely minimal given that each of these cases still meets the criteria for NCCN high-risk disease. Qualitative outcomes data such as toxicity events were recorded at the time of patient encounter in a standardized manner to ensure quality. Furthermore, grade ≥ 2 toxicity events, by definition, are typically associated with an intervention (invasive or non-invasive) which was recorded in each patient's medical record [Citation9]. This objective information was correlated with toxicity grading to help reduce the rate of underreported events. Secondly, our study involved a relatively small number of patients with a median follow-up time of only 3.7 years for patients receiving hypofractionated treatment to the prostate. While this length of follow-up may be adequate to detect differences in late GI toxicity [Citation20,Citation21], substantially longer follow-up will be required to determine if the different regimens have an effect on biochemical control. Lastly, biochemical freedom from progression was calculated from the beginning of ADT rather than its completion. Ideally, this would have been calculated from both the conclusion of ADT as well as the start. This aspect of treatment is often performed by physicians outside of our institution, with the conclusion of ADT less well-coordinated than its initiation. As we were unable to completely verify the appropriate dates of the conclusion of ADT we elected not to include this in our analysis. However, the overall rate of ADT between groups was similar (93% vs. 91%, p = 0.647) and we believe any differences caused by a difference in overall length of ADT between the two groups to be minimal.
The equivalent short-term biochemical freedom from progression and lack of worsened toxicity in the group receiving hRT appears to support that image-guided pelvic IMRT can be utilized to safely shorten the overall treatment time from nine weeks to less than six weeks for patients with high-risk prostate cancer.
Conclusion
Short-term follow-up indicates that image-guided pelvic IMRT with a hypofractionated simultaneous boost to the prostate appears to result in equivalent biochemical control compared to conventionally fractionation WPRT. We observed no statistically significant difference concerning biochemical control or early toxicity between these two regimens. The hypofractionated regimen was associated with fewer late rectal events. Continued follow-up of this patient population is important in order to observe if any other differences in outcomes between the regimens. Future prospective data should focus on providing confirmation of the roles of hypofractionation and elective nodal irradiation for the treatment of high-risk disease.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pollack A, Hanlon A, Horwitz E, Feigenberg S, Konski A, Movsas B, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionated dose escalation trial. Int J Rad Oncol Biol Phys 2006;64:518–52.
- Pahlajani N, Ruth K, Buyyounouski M, Chen D, Horwitz E, Hanks G, et al. Radiotherapy doses of 80 Gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate. Int J Rad Oncol Biol Phys 2012;82:1949–56.
- Leborgne F, Fowler J, Leborgne J, Mezzera J. Later outcomes and alpha/beta estimate from hypofractionated conformal three-dimensional radiotherapy versus standard fractionation for localized prostate cancer. Int J Rad Oncol Biol Phys 2012;82:1200–7.
- Shaffer R, Pickles T, Lee R, Moiseenko V. Deriving prostate alpha-beta ratio using carefully matched groups, long follow-up and the phoenix definition of biochemical failure. Int J Rad Oncol Biol Phys 2011;79:1029–36.
- Lawton C, DeSilvio M, Roach M, Uhl V, Kirsch R, Seider M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Rad Oncol Biol Phys 2007;69:646–55.
- Adkison J, McHaffie D, Bentzen S, Patel R, Khuntia D, Petereit D, et al. Phase I trial of pelvic nodal dose escalation with hypofractionated IMRT for high-risk prostate cancer. Int J Rad Oncol Biol Phys 2012;82:184–90.
- Quon H, Cheung P, Loblaw D, Morton G, Pang G, Szumacher E, et al. Hypofractionated concominant intensity-modulated radiotherapy boost for high-risk prostate cancer: Late toxicity. Int J Rad Oncol Biol Phys 2012; 82:898–905.
- Pollack A, Walker G, Buyyounouski M, Horwitz E, Price R, Feigenberg S, et al. Five year results of a randomized external beam radiotherapy hypofractionation trial for prostate cancer. Int J Rad Oncol Biol Phys 2011;81:S1.
- Common Terminology Criteria for Adverse Events (CTCAE) v4.0. US Department of Health and Human Services; 2009.
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley W, Sokol G, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Rad Oncol Biol Phys 2006;64:965–74.
- Roach M, DeSilvio M, Valicenti R, Grignon D, Asbell S, Lawton C, et al. Whole-pelvis, “mini-pelvis”, or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Rad Oncol Biol Phys 2006;66:647–53.
- Pommier P, Chabaud S, Lagrange J, Richaud P, Lesaunier F, Le Prise E, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol 2007;25:5366–73.
- Peeters S, Heemsbergen W, Koper P, van Putten W, Slot A, Dielwart M, et al. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990–6.
- Jacob R, Hanlon A, Horwitz, E, Movsas B, Uzzo R, Pollack A. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer 2004;100:538–43.
- Aizer A, Yu J, McKeon A, Decker R, Colberg J, Peschel R. Whole pelvic radiotherapy versus prostate only radiotherapy in the management of locally advanced or aggressive prostate adenocarcinoma. Int J Rad Oncol Biol Phys 2009;75: 1344–9.
- Mantini G, Tagliaferri L, Mattiucci G, Balducci M, Frascino V, Dinapoli N, et al. Effect of whole pelvic radiotherapy for patients with locally advanced prostate cancer treated with radiotherapy and long-term androgen deprivation. Int J Rad Oncol Biol Phys 2011;81:e721–6.
- Kupelian P, Reddy C, Carlson T, Altsman K, Willoughby T. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Rad Oncol Biol Phys 2002;53:904–12.
- McCammon R, Rusthoven K, Kavanagh B, Newell S, Newman F, Raben D. Toxicity assessment of pelvic intensity-modulated radiotherapy with hypofractionated simultaneous integrated boost to prostate for intermediate- and high-risk prostate cancer. Int J Rad Oncol Biol Phys 2009;75: 413–20.
- Reddy K, Nelson B, McCammon, Newell S, Newman F, Raben D. Preliminary outcomes for the treatment of intermediate- and high-risk prostate cancer patients using pelvic intensity modulated radiotherapy with hypofractionated simultaneous integrated boost to prostate. Int J Rad Oncol Biol Phys 2010;78:S376.
- Arcangeli G, Fowler J, Gomellini S, Arcangeli S, Saracino B, Petrongari M, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three- dimensional conformal radiotherapy for prostate cancer. Int J Rad Oncol Biol Phys 2011;79:1013–21.
- Kupelian P, Willoughby T, Reddy C, Klein E, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Rad Oncol Biol Phys 2007;68:1424–30.