Abstract
Background. The multitargeted tyrosine kinase inhibitor sunitinib is used in various cancers. Clinical studies have reported a substantial variation in the incidence of hypothyroidism associated with sunitinib, without a systemic attempt to synthesize these data. Methods. We searched Medline databases for relevant clinical trials published up to May 2012. Phase II and III trials and expanded access programs of sunitinib in patients with any type of cancer that reported occurrence of hypothyroidism were eligible. The summary incidence, relative risk (RR) and 95% confidence intervals (CIs) were calculated using random- or fixed-effects models based on the heterogeneity of included studies. Results. Incidence analysis was performed using 6678 sunitinib-treated patients from all 24 eligible trials. The incidence of all- and high-grade hypothyroidism was 9.8% (95% CI 7.3–12.4%) and 0.4% (95% CI 0.3–0.5%), respectively. A meta-analysis of seven randomized trials with 2787 subjects revealed a RR of all- and high-grade hypothyroidism of 13.95 (95% CI 6.91–28.15; p < 0.00001) and 4.78 (95% CI 1.09–20.84; p = 0.04), respectively. Subgroup analysis revealed a significantly higher incidence of all-grade hypothyroidism in patients receiving sunitinib for longer duration than in patients receiving sunitinib for shorter duration (p = 0.02). Conclusions. This meta-analysis of data available from clinical trials demonstrates that sunitinib is associated with a significant risk of developing all- and high-grade hypothyroidism. These data provide further evidence to recommend monitoring for hypothyroidism in patients receiving sunitinib.
Sunitinib is a rationally designed small molecule with structural similarity to adenosine triphosphate (ATP) that prevents activation of a broad-spectrum of tyrosine kinases (TKs). Dysregulation of signaling pathways mediated by TKs is involved in the pathogenesis of a wide variety of cancers [Citation1]. Sunitinib targets vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), FMS-like tyrosine kinase-3 (FLT3) and stem cell factor receptor (c-Kit) [Citation2]. Currently, sunitinib is approved by the FDA for patients with metastatic renal cell carcinoma (RCC), imatinib-resistant GI stromal tumor (GIST) and advanced pancreatic neuroendocrine tumor (PNET) [Citation3–5].
The toxicity profiles resulting from VEGF- targeted tyrosine kinase inhibitors (TKIs), including sunitinib and sorafenib are unique and different from traditional cytotoxic chemotherapeutic agents. For instance, previous studies have demonstrated an increased risk of developing hypertension, hand-foot skin reaction, bleeding, arterial thromboembolism and hematologic toxicities [Citation6–13]. Hypothyroidism associated with sunitinib has been reported with a substantial variation in the incidences, ranging from 0 to 14% in randomized controlled trials [Citation3–5, Citation14–17]. Additionally, prospective observational studies showed an even higher incidence of hypothyroidism in patients treated with sunitinib, varying from 27% to 85% [Citation18–22]. There has been no systematic attempt to synthesize these data and the overall risk of hypothyroidism with sunitinib has yet to be defined. It is important to fully recognize the risk of sunitinib-induced hypothyroidism, because hypothyroidism affects the quality of life and can be effectively managed with thyroid hormone replacement if it is correctly diagnosed. Therefore, the aim of this study was to investigate the incidence and risk of developing hypothyroidism in patients treated with sunitinib by performing a systemic review and meta-analysis of available clinical trials.
Methods
Study selection
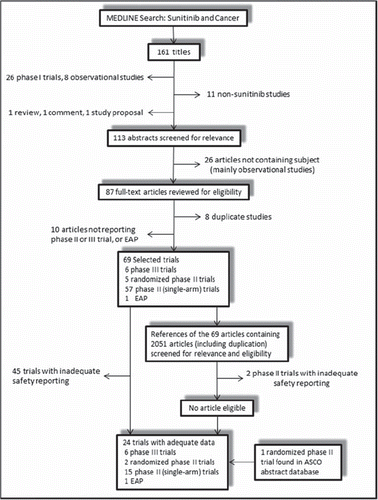
We performed an independent review of Medline databases from January 1966 to May 2012. The key words and Medical Subject Headings used for the search were ‘‘sunitinib’’ and ‘‘cancer’’. The search was restricted to human studies, clinical trials and English language. Identified studies were screened by their titles and abstracts for relevance. Phase I trials were excluded because of the different drug dosages as well as the small number of patients in these trials (). Studies meeting the following criteria were included: phase II or III trials, or expanded access programs (EAPs) in patients with cancer, patients assigned to treatment with sunitinib, and safety data available for all- or high-grade (≥ grade 3) hypothyroidism events based on the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute. Studies that reported only thyroid function monitoring without mention of hypothyroidism events were also eligible as long as the corresponding author could provide the data. Only the most recent or complete report of clinical trials was included when duplicate publications were identified. References in relevant reports were reviewed manually. The most recent package insert was also reviewed to identify relevant information [Citation23]. Independent reviewers (T.F and Y.J.S.) retrieved full texts of the relevant articles to assess eligibility. There was complete agreement between the two investigators on the final results. An independent search using Embase and Cochrane electronic databases was also conducted to ensure that there were no additional studies. We manually searched all the abstracts containing the same search terms from the American Society of Clinical Oncology (ASCO) conferences held between January 2006 and May 2012. The selection and systematic review of trials was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation24].
Data extraction and clinical end points
For each study, the following information was obtained independently by the two investigators: first author's name, year of publication, trial phase, underlying malignancy, number of patients enrolled, treatment arms, median age, median treatment duration, median progression free survival (PFS), number of patients available for analysis, number of hypothyroidism events, thyroid function monitoring and version of CTCAE (). Any discrepancies between reviewers were resolved by consensus. Adverse events of all- and high-grade (≥ grade 3), as defined by the CTCAE, were extracted for analysis. The CTCAE (version 3) defines the grading of hypothyroidism as: grade I, asymptomatic, intervention not indicated; grade II, symptomatic, not interfering with activities of daily living (ADL); thyroid replacement indicated; grade III, symptoms interfering with ADL; hospitalization indicated; and grade IV, life-threatening myxedema coma [Citation25].
Table I. Characteristics of the trials included in the meta–analysis.
Statistical analysis
The principal summary measures were incidence, relative risk (RR) and corresponding 95% CIs of all-grade (Grade 1–4) and high-grade (Grade 3 and 4) hypothyroidism. For the calculation of incidence, the episodes of hypothyroidism and the number of patients receiving sunitinib were extracted from the safety profiles of single-arm and randomized controlled clinical trials. The proportion of patients with adverse events and 95% CIs were derived from each trial. We calculated the RRs and CIs with data extracted only from randomized controlled studies, comparing the incidence of hypothyroidism in patients assigned to sunitinib with those assigned to control treatment in the same trial. To calculate the 95% CIs, the variance of a log-transformed study-specific RR was derived using the delta method. Statistical heterogeneity between trials included in the meta-analysis was assessed using Cochrane’s Q statistic, and inconsistency was quantified with I2 statistic. Assumption of homogeneity was considered invalid for p-values less than 0.1. Summary incidence and RRs were calculated using random- or fixed-effects models depending on the heterogeneity of included studies. When substantial heterogeneity was not observed, the pooled estimate calculated based on the fixed-effects model was reported using inverse variance method. When substantial heterogeneity was observed, the pooled estimate calculated based on the random-effects model was reported using the DerSimonian method, which considers both within- and between-study variations. We evaluated publication bias using funnel plots and with Begg's and Egger's tests. A two-tailed p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Review Manager 5.1 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) and Comprehensive Meta Analysis program (Version 2, Biostat, Englewood, NJ, USA).
Results
Search results and population characteristics
The search strategy yielded a total of 161 potentially relevant articles on sunitinib for inclusion in the study. A total of 138 citations were excluded. outlines the selection process and reasons for study exclusion. Two phase III trials reporting thyroid function monitoring without hypothyroidism event mentioned were included because Pfizer provided the data on hypothyroidism events [Citation15,Citation16]. One phase II trial was excluded because reported hypothyroidism events included patients who presented with baseline hypothyroidism [Citation26]. We included an additional full-text publication identified through manual review of ASCO conferences abstracts [Citation27].
At the end of this review process, 24 trials were eligible for analysis (i.e. phase II or III studies or EAPs) [Citation3–5,Citation14–16,Citation27–43] 15 phase II trials were single-arm studies and two phase II studies were randomized trials. A total of 8014 patients were included for the meta-analysis; 5498 of these patients had RCC, and 2516 had other malignancies. Sunitinib was administered at 37.5 mg daily (continuous daily dosing) in 11 studies and at 50 mg once daily on a 4-weeks-on/2-weeks-off schedule (intermittent dosing) in 11 studies. Bergh et al. administered sunitinib 37.5 mg once daily on a 2-weeks-on/1-weeks-off schedule [Citation15] and Motzer et al. compared sunitinib 37.5 mg daily with sunitinib 50 mg daily on a 4-weeks-on/2-weeks-off schedule in a randomized, phase II trial [Citation27]. Patients with uncontrolled hypothyroidism were generally excluded from these trials.
Overall incidence of hypothyroidism
For the incidence analysis, a total of 6678 patients from both randomized and non-randomized studies (six phase III, 17 phase II trials and one EAP) were considered. In total 6660 patients were included for all-grade hypothyroidism events, and 6678 were included for high-grade events. This numerical difference was attributable to the fact that one trial reported only high-grade events and the others reported both all- and high-grade events. All-grade hypothyroidism occurred in 504 of 6660 patients, conferring an incidence of 9.8% (95% CI 7.3–12.4%) (). The test for heterogeneity was significant for all-grade hypothyroidism (Q = 412.07; p < 0.001; I2 = 94.7%) and the random-effects model was used. High-grade hypothyroidism occurred in 28 of 6678 patients, conferring an incidence of 0.4% (95% CI 0.3–0.5%). The test for heterogeneity was not significant (Q = 14.75; p = 0.903; I2 = 0.0%) and the fixed-effects model was used.
Table II. Incidence of all- and high-grade hypothyroidism events from all included trials.
Relative risk of hypothyroidism
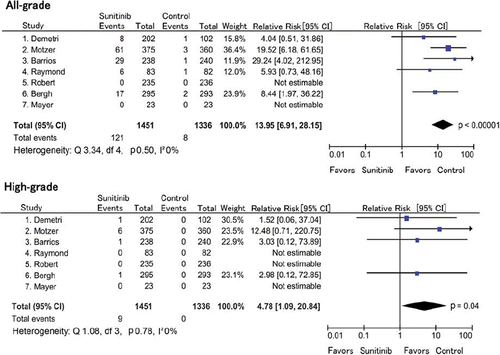
For the RR analysis, seven randomized trials with a placebo or a control arm without sunitinib were available (six phase III studies and one phase II study, representing 2787 patients). In four trials [Citation4,Citation5,Citation15,Citation17], the RR of all-grade hypothyroidism was not statistically significant, while sunitinib was associated with a significant risk of hypothyroidism in three trials [Citation3,Citation14,Citation16], creating a major discrepancy. In addition, the RR with sunitinib for high-grade hypothyroidism was not statistically significant in any individual trial. The meta- analysis showed a RR of all- and high-grade hypothyroidism of 13.95 (95% CI 6.91–28.15; p < 0.00001) and 4.78 (95% CI 1.09–20.84; p = 0.04), respectively (). The fixed-effects model was used because there was no significant heterogeneity observed in the RR analysis of all-grade hypothyroidism events (Q = 3.44; p = 0.49; I2 = 0%) or high-grade hypothyroidism events (Q = 1.08; p = 0.78; I2 = 0%).
Subgroup analyses
As an exploratory analysis, we evaluated the incidence and RR of all- and high-grade hypothyroidism events based on the underlying malignancy (RCC vs. non-RCC), the thyroid function monitoring schedule (trials with vs. without regular monitoring), the sunitinib dosing schedule (continuous daily dosing vs. intermittent dosing) and the duration of treatment with sunitinib (trials with long vs. short treatment duration). The patients enrolled in trials with longer treatment duration than the mean treatment duration of all trials (3.5 months) demonstrated a significantly higher incidence of all-grade hypothyroidism compared with those enrolled in trials with shorter longer treatment duration (p = 0.02). There was no statistically significant difference between the other subgroups ().
Table III. Relative risk and incidence of all- and high-grade hypothyroidism events stratified by underlying malignancy, monitoring, sunitinib dosing schedule or duration of treatment with sunitinib.
Publication bias
We found no evidence of publication bias for relative risk of all- and high-grade hypothyroidism by either the Egger's or the Begg's test (p > 0.05). The Egger's test suggested evidence of publication bias for incidence of all-grade hypothyroidism, while the Begg's tests showed no evidence of bias (p > 0.05). This difference in the results obtained from the two methods may be due to a greater statistical power of the Egger test [Citation44]. Evidence of publication bias was only observed by both the Egger's and the Begg's test for incidence of high-grade hypothyroidism (p < 0.05).
Discussion
To our knowledge, this is the first meta-analysis to evaluate the risk of hypothyroidism in patients treated with the tyrosine kinase inhibitor (TKI) sunitinib. Our analysis of data from phase II and III randomized controlled trials showed a significant increased risk of all- and high-grade hypothyroidism with this agent. This study also demonstrated the overall incidence of hypothyroidism (all-grade: 9.8%, high-grade: 0.4%) by the analysis of published phase II and III trials and expanded access programs of sunitinib.
The incidence of all-grade hypothyroidism in our analysis is lower than the 27% to 85% range in prospective observational studies [Citation18–22]. First, this likely reflects the fact that thyroid function tests (TFTs) were obtained only from symptomatic patients in most of the trials included in our analysis while the prospective observational studies checked TFTs routinely. Second, the shorter (3.5 months) average duration of sunitinib treatment among the trials included our study compared with 7.3–9.3 months in the prospective observational studies [Citation18,Citation20], may explain our finding of a lower incidence of hypothyroidism. Desai et al. reported that the risk for hypothyroidism increased with duration of sunitinib therapy [Citation18]. And we revealed a significantly higher incidence of all-grade hypothyroidism in patients with longer treatment duration. Finally, the definition of hypothyroidism may have affected the incidence rate. The trials in our meta-analysis utilized the Criteria for Adverse Events (CTCAE) based on the presence of symptoms and need for thyroid replacement [Citation25], without reporting criteria for TFT abnormalities. The use of TFTs in prospective observational studies may have resulted in better recognition and higher incidence of mild or subclinical hypothyroidism. Interestingly, Feldman et al. only obtained TFTs in symptomatic patients enrolled in prospective clinical trials with sunitinib for metastatic renal cell carcinoma and observed a lower incidence of hypothyroidism (18%) [Citation45].
Several potential mechanisms of sunitinib- induced thyroid dysfunction have been proposed. Desai et al. suggested destructive thyroiditis because of the high incidence of transient thyrotoxicosis prior to developing hypothyroidism [Citation18]. However, a number of other studies have shown recovery of TSH values to the normal range following treatment with sunitinib, which does not support the hypothesis of destructive thyroiditis [Citation20,Citation46]. Mannavola et al. proposed inhibition of iodide uptake based on clinical studies of thyroid radioiodine uptake [Citation19], but in vitro studies showed no impairment of iodide uptake [Citation47]. Wong et al. showed that sunitinib impairs peroxidase activity in vitro [Citation48], but this has not yet been confirmed in vivo. Doreen Braun et al. showed that sunitinib non-competitively inhibited thyroid hormone transport mediated by the transmembrane transporter MCT8 in vitro. However, the therapeutic plasma level of sunitinib is one order of magnitude below the in vitro IC50 [Citation49]. Since sunitinib inhibits VEGF receptors as a principal mechanism of its action on tumors, regression of the thyroid vascular bed induced by VEGF inhibition is the likely explanation. Supporting evidence for this theory includes mouse studies that have shown glandular capillary regression with TKI exposure [Citation50]. Makita et al. reported thyroid volume and blood flow were both reduced on sunitinib compared to values obtained when sunitinib was stopped [Citation51]. It has been postulated that rapid reduction in thyroid cell perfusion could result in thyroiditis, leading to a transient thyrotoxicosis and a slower decrease in the blood flow could cause gradual thyroid destruction, resulting in hypothyroidism. Decreased blood flow may also explain the reduced glandular uptake of radioiodine [Citation52]. However, Mannavola et al. did not detect changes in thyroid volume with ultrasonography or vascularity with echo-color Doppler between before and during sunitinib treatment [Citation19]. Further studies are needed to uncover the mechanism of sunitinib-induced hypothyroidism.
Correlations between efficacy of sunitinib in patients with advanced renal cell carcinoma (RCC) and hypothyroidism were reported. First, in the Wolter et al. study of sunitinib for advanced RCC, median progression-free survival (PFS) was respectively 10.3 and 3.6 months with and without thyroid function abnormalities (p = 0.047) [Citation53]. Second, Schmidinger et al. showed that patients with subclinical hypothyroidism during sunitinib or sorafenib treatment had a statistically significant improvement of overall survival (not reached in hypothyroid patients vs. 13.9 months in euthyroid patients; p = 0.016) [Citation54]. Finally, Riesenbeck et al. studied patients with metastatic RCC who received sunitinib or sorafenib. Hypothyroidism was associated with a longer PFS (16.0 months vs. 6.0 months, p = 0.032) [Citation55]. A possibility thyroid hormone may be permissive for tumor growth has been noted but remains controversial [Citation56]. Interestingly, the application of levothyroxine did not have an influence on survival in these patients [Citation54,Citation55]. However, Sabatier et al. showed that among RCC patients treated with sunitinib, PFS between hypothyroid patients with thyroid hormone replacement was not significantly different from patients without thyroid dysfunction (18.9 and 15.9 months, p = 0.94) [Citation57]. Therefore, it is still unclear whether hypothyroidism is a biomarker for efficacy of sunitinib in patients with cancer or a hypothyroid state is associated with improved outcomes.
Management of sunitinib-associated hypothyroidism remains controversial. Most authors agree that any preexisting hypothyroidism should be detected and treated before starting sunitinib treatment [Citation58]. The most recent package insert recommends thyroid function should be checked when patients develop signs and/or symptoms suggestive of thyroid dysfunction [Citation23]. Based on our finding here, we suggest that thyroid function should be monitored regularly during treatment with sunitinib. However, the frequency at which TSH should be measured is unknown. For patients receiving sunitinib on a 4-week ON/2-week OFF schedule, Wolter et al. proposed measuring TSH on day 1 and 28 of the first four cycles, then on day 28 of every third cycle [Citation20]. In contrast, Torino et al. propose measurement of TSH on day 1 of every cycle of sunitinib treatment [Citation59]. Checking serum TSH concentration on day 1 of each cycle has also been suggested by Illouz et al. [Citation60].
Although thyroid function test abnormalities caused by sunitinib are not always of clinical importance, serious complications such as myxedema coma and metabolic adverse effects such as hypercholesterolemia have been reported with sunitinib [Citation19,Citation45]. In most patients, hypothyroidism can be controlled with thyroid hormone [Citation18,Citation20]. Therefore, starting thyroid hormone replacement in the setting of sunitinib-induced overt hypothyroidism is generally accepted. A ‘safe’ serum TSH level remains to be defined because no prospective studies are available to evaluate the advantage of treatment of subclinical hypothyroidism in patients treated with sunitinib. As recommended by American Thyroid Association guidelines, initiating thyroid hormone when TSH rises to 10 mIU/ml or between 5 and 10 mIU/ml with clinical symptoms that can be linked to hypothyroidism, may be warranted until further clinical evidence becomes available [Citation61]. A potential problem with this algorithm is the difficultly in attributing non-specific symptoms such as fatigue and hair and skin changes to hypothyroidism. These symptoms could be due to an adverse event of sunitinib independent of thyroid dysfunction or underlying cancer itself [Citation22,Citation62].
The results described here are affected by the limitations of individual clinical trials that were included in this meta-analysis. First, baseline thyroid function was not mentioned in all of these trials. This could lead to an overestimation of new onset hypothyroidism. However, this is unlikely because the incidence of all-grade hypothyroidism of 4.0% (95% CI 1.0–7.0%) in patients who received the control treatment in our meta-analysis was at the low end of the baseline frequency in an adult population of 4–8% [Citation61]. In addition, it is doubtful that the baseline thyroid function biased the relative risk because it was calculated using randomized controlled clinical trials, with direct comparison with and without sunitinib. Second, evidence of publication bias was observed for the incidence of high-grade hypothyroidism by both the Egger's and the Begg's test. This might be related to the large number of trials included in the analysis, the inclusion of small studies and the between-trial heterogeneity. The main concern is whether the existence of small-study effects would affect the conclusions of our study. Cumulative meta-analysis plots of high-grade hypothyroidism did not show shift in the cumulative effect size adding smaller studies (data not shown). This suggests that our results are not biased by smaller studies.
In conclusion, this study demonstrates that sunitinib, a multi-tyrosine kinase inhibitor, is associated with a significant risk of developing all- and high-grade hypothyroidism. This study provides further evidence for routine thyroid function monitoring in patients treated with sunitinib to detect hypothyroidism at an early stage and treat it properly in order to prevent serious complications.
Acknowledgments
The authors would like to thank Kamyar Arasteh for statistical analysis support, Ronald H. Blum for critically reading the manuscript and Adrienne M. Fleckman for editorial assistance to the authors during the revision of this manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. New Engl J Med 2005;353: 172–87.
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248 , a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327–37.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329–38.
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27:3584–90.
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl J Med 2011;364:501–13.
- Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol 2009;48: 9–17.
- Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol 2008;9: 117–23.
- Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: A meta-analysis. Clin Genitourin Cancer 2009;7:11–9.
- Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta- analysis. Acta Oncol 2008;47:176–86.
- Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic review and meta- analysis of clinical trials. Lancet Oncol 2009;10:967–74.
- Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. J Clin Oncol 2010;28:2280–5.
- Schutz FA, Je Y, Choueiri TK. Hematologic toxicities in cancer patients treated with the multi-tyrosine kinase sorafenib: A meta-analysis of clinical trials. Crit Rev Oncol Hematol 2011;80:291–300.
- Nalluri SR, Su X, Shah R, Wu S. Risk of cytopenia with sunitinib in patients with renal cell cancer and non-RCC cancers: A meta-analysis. In: Proceedings of the 2010 Genitourinary Cancers Symposium, ASCO, abstract 393. San Francisco, California: ASCO.
- Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2- negative advanced breast cancer. Breast Cancer Res Treat 2010;121:121–31.
- Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: A phase III, randomized, open-label trial. Clin Breast Cancer 2011;11:82–92.
- Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: Results of a prospective, randomized phase III study. J Clin Oncol 2012;30:921–9.
- Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, et al. SABRE-B: An evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol 2010;21:2370–6.
- Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 2006;145:660–4.
- Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab 2007;92: 3531–4.
- Wolter P, Stefan C, Decallonne B, Dumez H, Bex M, Carmeliet P, et al. The clinical implications of sunitinib- induced hypothyroidism: A prospective evaluation. Br J Cancer 2008;99:448–54.
- Sabatier R, Gravis G, Deville J, Salem N, Brunelle S, Walz J, et al. Hypothyroidism and survival during sunitinib therapy in metastatic renal cell cancer: A prospective observational analysis. In: Proceedings of the 2009 Genitourinary Cancers Symposium, ASCO, abstract 317. Orlando, Florida: ASCO.
- Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2007; 99:81–3.
- Pfizer: Sutent prescribing information. Available from: http://www.pfizer.com/files/products/uspi_sutent.pdf
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–12.
- National Cancer Institute: Common Toxicity Criteria version 3. Available from: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer 2012;118:1252–9.
- Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 2012;30:1371–7.
- Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 2008;26:3743–8.
- Kontovinis LF, Papazisis KT, Touplikioti P, Andreadis C, Mouratidou D, Kortsaris AH. Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: Clinical outcomes and plasma angiogenesis markers. BMC Cancer 2009;9:82.
- George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959–68.
- George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 2009;27:3154–60.
- Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: An expanded-access trial. Lancet Oncol 2009;10:757–63.
- Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, Nikolaidou M, Bobos M, Calderaro J, et al. A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol 2010;65:649–60.
- Mackay HJ, Tinker A, Winquist E, Thomas G, Swenerton K, Oza A, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol 2010;116:163–7.
- Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol 2010;28:21–8.
- Koeberle D, Montemurro M, Samaras P, Majno P, Simcock M, Limacher A, et al. Continuous sunitinib treatment in patients with advanced hepatocellular carcinoma: A Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist 2010;15:285–92.
- Shanafelt T, Zent C, Byrd J, Erlichman C, Laplant B, Ghosh A, et al. Phase II trials of single-agent anti-VEGF therapy for patients with chronic lymphocytic leukemia. Leuk Lymph 2010;51:2222–9.
- Ping G, Hui-Min W, Wei-Min W, Bao-Hui H. Sunitinib in pretreated advanced non-small-cell lung carcinoma: A primary result from Asian population. Med Oncol 2011;28: 578–83.
- Biagi JJ, Oza AM, Chalchal HI, Grimshaw R, Ellard SL, Lee U, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: An NCIC Clinical Trials Group Study. Ann Oncol 2011;22:335–40.
- Buckstein R, Kuruvilla J, Chua N, Lee C, Macdonald DA, Al-Tourah AJ, et al. Sunitinib in relapsed or refractory diffuse large B-cell lymphoma: A clinical and pharmacodynamic phase II multicenter study of the NCIC Clinical Trials Group. Leuk Lymph 2011;52:833–41.
- Schneider BJ, Gadgeel SM, Ramnath N, Wozniak AJ, Dy GK, Daignault S, et al. Phase II trial of sunitinib maintenance therapy after platinum-based chemotherapy in patients with extensive-stage small cell lung cancer. J Thorac Oncol 2011;6:1117–20.
- Novello S, Camps C, Grossi F, Mazieres J, Abrey L, Vernejoux JM, et al. Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J Thorac Oncol 2011;6:1260–6.
- Gervais R, Hainsworth JD, Blais N, Besse B, Laskin J, Hamm JT, et al. Phase II study of sunitinib as maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer. Lung Cancer 2011;74:474–80.
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29.
- Feldman DR, Martorella AJ, Robbins RJ, Motzer RJ. Re: Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2007;99:974–5; author reply 6–7.
- Grossmann M, Premaratne E, Desai J, Davis ID. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol 2008;69:669–72.
- Salem AK, Fenton MS, Marion KM, Hershman JM. Effect of sunitinib on growth and function of FRTL-5 thyroid cells. Thyroid 2008;18:631–5.
- Wong E, Rosen LS, Mulay M, Vanvugt A, Dinolfo M, Tomoda C, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 2007;17:351–5.
- Braun D, Kim TD, le Coutre P, Kohrle J, Hershman JM, Schweizer U. Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab 2012;97:E100–5.
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 2006;290:H560–76.
- Makita N, Miyakawa M, Fujita T, Iiri T. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid 2010;20:323–6.
- Hershman JM, Liwanpo L. How does sunitinib cause hypothyroidism?Thyroid 2010;20:243–4.
- Wolter P, Stefan C, Decallonne B, Dumez H, Fieuws S, Wildiers H, et al. Evaluation of thyroid dysfunction as a candidate surrogate marker for efficacy of sunitinib in patients with advanced renal cell carcinoma. J Clin Oncol 2008;26:280s (Abstract 5126).
- Schmidinger M, Vogl UM, Bojic M, Lamm W, Heinzl H, Haitel A, et al. Hypothyroidism in patients with renal cell carcinoma: Blessing or curse?Cancer 2011;117:534–44.
- Riesenbeck LM, Bierer S, Hoffmeister I, Kopke T, Papavassilis P, Hertle L, et al. Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J Urol 2011;29: 807–13.
- Garfield D, Hercbergs A, Davis P. Unanswered questions regarding the management of sunitinib-induced hypothyroidism. Nature Clin Pract Oncol 2007;4:674–52.
- Sabatier R, Eymard JC, Walz J, Deville JL, Narbonne H, Boher JM, et al. Could thyroid dysfunction influence outcome in sunitinib-treated metastatic renal cell carcinoma?Ann Oncol 2012;23:714–21.
- Eisen T, Sternberg CN, Robert C, Mulders P, Pyle L, Zbinden S, et al. Targeted therapies for renal cell carcinoma: Review of adverse event management strategies. J Natl Cancer Inst 2012;104:93–113.
- Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nature Rev Clin Oncol 2009;6:219–28.
- Illouz F, Laboureau-Soares S, Dubois S, Rohmer V, Rodien P. Tyrosine kinase inhibitors and modifications of thyroid function tests: A review. Eur J Endocrinol 2009; 160:331–6.
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA 2004;291:228–38.
- Wolter P, Dumez H, Schoffski P. Sunitinib and hypothyroidism. New Engl J Med 2007;356:1580; author reply 1581.