To the Editor,
Oxaliplatin is a platinum-based antineoplastic agent that is widely used in chemotherapy for various cancer types due to efficacy and manageable toxicity profiles. Side effects of oxaliplatin are now well-described, including hypersensitivity reaction, neutropenia, and peripheral neuropathy. To date, a possible association between Torsades de Pointes (TdP) with oxaliplatin has never previously been described though one case report linked a long QT syndrome (LQTS) and oxaliplatin. We present a case of oxaliplatin-induced TdP and LQTS in a gastric cancer patient. TdP is a specific, rare form of polymorphic ventricular tachycardia (VT) that exhibits distinct characteristics on electrocardiogram. Patients with TdP may present with syncope or even sudden cardiac death. This case highlights that TdP and LQTS in gastric cancer patient fully recovered and showed normalized QT interval after discontinuation of oxaliplatin. Patients with cancer are at increased risk of LQTS because of the high prevalence of predisposing risk factors such as electrolyte abnormalities, poor oral intake, and concomitant medications, therefore the use of oxaliplatin needs to be properly evaluated in each patient to reduce the risk of LQTS and TdP.
Case report
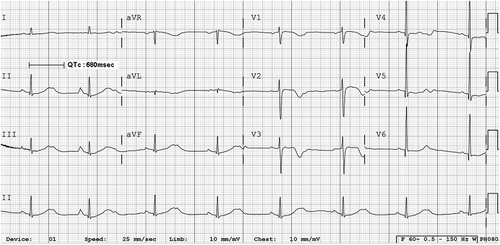
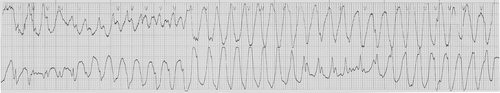
A 72-year-old male patient with gastric cancer developed polymorphic VT named TdP and LQTS after administration of oxaliplatin-containing chemotherapy. He experienced recurrent episodes of syncope after two cycles of combination chemotherapy with oxaliplatin and 5-fluorouracil (5-FU). Chemotherapy regimen was modified FOLFOX consisting of oxaliplatin 85 mg/m2 iv day 1, 5-FU 400 mg/m2 iv bolus day 1, 5-FU 1500 mg/m2 22 h continuous infusion day 1, 2, Leucovorin (folinic acid) 75 mg/m2 22 h continuous infusion day 1, 2, every two weeks. Before chemotherapy, the QT interval corrected for heart rate using Bazett formula was 467 ms, which was slightly increased, but it was in borderline range (430–470 ms) [Citation1]. There was no immediate complication during infusion of chemotherapy. He visited the outpatient clinic seven days after discharge with a chief complaint of recurrent episodes of syncope. The duration of syncope was about 10 s and the frequency was two or three times a day. The associated symptoms with syncope were urinary incontinence and sweating. There was neither headache nor focal neurologic deficit at any time. He was immediately admitted to the cardiac intensive care unit for evaluation and management of syncope. Twenty-four hour ECG and blood pressure monitoring was started promptly. On admission, his ECG showed QT prolongation and 2:1 atrioventricular block with a heart rate of 48/min; the QTc interval was 680 ms (), which was markedly increased compared to that before chemotherapy (QTc: 467ms). He developed three episodes of syncope during the initial 24 h. While the patient was experiencing syncope, TdP was noted on the ECG monitoring (). It lasted for 15 s, and spontaneously came to an end to baseline rhythm without medical intervention. On ECG analysis, intermittent ventricular premature contraction triggered the TdP with the R on T phenomenon. We inserted a temporary pacemaker through femoral vein and controlled the heart rate up to 80/min to prevent the bradycardia associated R on T phenomenon. He had no history of diabetes mellitus, hypertension, hypercholesterolemia and ischemic heart disease. He was not under any medication that would cause LQTS except oxaliplatin. The levels of serum magnesium, calcium and potassium were normal. Echocardiography revealed normal systolic and diastolic function without any evidence of ischemic heart disease. We stopped further chemotherapy with oxaliplatin. The QTc interval was slowly normalized (QTc: 461 ms) and TdP did not recur again, so we removed the temporary pacemaker after five days. After discontinuation of chemotherapy, he did not experience the syncope again and was discharged without any specific antiarrythmic drug.
Discussion
We reported a case of oxaliplatin-induced LQTS and TdP. TdP is a French term that literally means twisting of the spikes. In 1966, Francois Dessertenne first described this specific, rare form of polymorphic VT with a characteristic twisting of the QRS complex around the isoelectric baseline [Citation2]; TdP is associated with a fall in arterial blood pressure, which can lead to syncope. If TdP is rapid or prolonged, it can lead to ventricular fibrillation and sudden cardiac death. Essentially, TdP can be caused by either congenital or acquired LQTS. Several drugs including antiarrythmic drugs and psychiatric drugs have been associated with the development of LQTS and TdP, and some common causes of LQTS and TdP include diarrhea, hypomagnesemia and hypokalemia [Citation3]. In the present case, the patient did not have congenital LQTS because his QTc interval was normal before chemotherapy. And, he did not take any medications that could act as probable causes of LQTS before the chemotherapy.
The FOLFOX regimen is commonly used among gastric cancer and colorectal cancer patients due to its efficacy and tolerable toxicity profiles. Ng et al. reported the observed frequency and pattern of cardiotoxicity among colorectal cancer patients treated with capecitabine (oral 5-FU) and oxaliplatin [Citation4]. In total 6.5% of patients (10/153) developed cardiac events. The study focused on the cardiotoxicity of capecitabine instead of the side effects of oxaliplatin. The cardiotoxicity of oral 5-FU, capecitabine was mostly angina pectoris [Citation4] as well as the one of intravenous 5-FU [Citation5,Citation6], but Ng et al. suggested that oxaliplatin is associated with cell membrane channelopathies which could predispose patients to arrhythmia [Citation4]. The cardiotoxicity of both intravenous 5-FU and oral 5-FU was relatively well known, but LQTS and TdP was never been reported. Oxaliplatin differed from 5-FU in causing LQTS and TdP, therefore oxaliplatin-induced LQTS and TdP should be separated from 5-FU-induced angina, but it is uncertain whether the combination of oxaliplatin and 5-FU would enhance the cardiotoxicity of each drug.
Oxaliplatin may result in LQTS because it can alter sodium channel kinetics [Citation7,Citation8]. The association between oxaliplatin and LQTS was first reported by Woei Chung et al. [Citation9]. They reported LQTS and syncope immediately after oxaliplatin infusion, but it did not generate into TdP, which was different from our present case. In this case, TdP with LQTS were not infusion-related immediate side effects, but they were delayed ones because they occurred seven days after administration of oxaliplatin. Of 12,107 patients who reported side effects of oxaliplatin on the eHealthMe website where continuous monitoring is done for drug-related adverse effects, eight people (0.07%) reported TdP (mostly within the first one month of starting the drug) [Citation10]. The cardiac toxicity of oxaliplatin may be underestimated since it is frequently used with other chemotherapeutic agents with established cardiac toxicity such as 5-FU. Physicians should be aware of LQTS and TdP as rare side effects of oxaliplatin, and hypokalemia and hypomagnesemia should be corrected before administration of oxaliplatin. The QT interval should be closely monitored on a regular basis before and after administration of oxaliplatin so that TdP can be prevented. Particularly, awareness of these rare side effects of oxaliplatin is vital to reduce risks in vulnerable cancer patients with predisposing risk factors such as electrolytic abnormalities, anorexia and concomitant medications.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Goldenberg I, Moss AJ, Zareba W. QT interval: How to measure it and what is “normal”. J Cardiovasc Electrophysiol 2006;17:333–6.
- Dessertenne F. Ventricular tachycardia with 2 variable opposing foci. Arch Mal Coeur Vaiss 1966;59:263–72.
- Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J 2007;153:891–9.
- Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer 2005;41:1542–6.
- de Forni M, Malet-Martino MC, Jaillais P, Shubinski RE, Bachaud JM, Lemaire L, et al.Cardiotoxicity of high-dose continuous infusion fluorouracil: A prospective clinical study. J Clin Oncol 1992;10:1795–801.
- Akhtar SS, Salim KP, Bano ZA. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: A prospective study. Oncology 1993;50:441–4.
- Keller GA, Ponte ML, Di Girolamo G. Other drugs acting on nervous system associated with QT-interval prolongation. Curr Drug Saf 2010;5:105–11.
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol 2000;406:25–32.
- Woei Chung L, Liao YM, Hsieh CY, Lin CY. Oxaliplatin-induced long QT syndrome in a patient with appendiceal adenocarcinoma. Acta Oncol 2009;48:156–7.
- eHealtheMe. Could oxaliplatin cause Torsade de pointes? Real World Drug outcomes. 2012. Available from: http://www.ehealthme.com/ds/oxaliplatin/torsade+ de+ points [cited 2012 December 24].