Abstract
Background. Hypoxia up-regulated expression of tissue factor (TF) may facilitate tumor cell metastasis, but transcriptional mechanisms remain undefined. Material and methods. To verify the role of Egr-1 in hypoxia-induced TF expression in breast cancer cells, quantitative PCR and Western blot analysis were performed. The secretion of VEGF under hypoxia was detected by enzyme-linked immunosorbent assay (ELISA). Egr-1 and HIF-1α siRNA were transiently transfected into breast cancer cells to evaluate their specific roles. Results. The increased Egr-1 expression occurring in hypoxic breast cancer cells can up-regulate TF expression and stimulating protein 1(Sp1) was not responsible for the hypoxia-induced expression of TF. HIF-1α mediated the hypoxia-induced up-regulation of TF expression through vascular endothelial growth factor (VEGF). The regulatory effects of Egr-1 on TF under hypoxia were independent of HIF-1α. Either Egr-1 or HIF-1α was responsible for hypoxic induction of tumor cells adhesion. HIF-1α, but not Egr-1, had a pivotal role in human breast cancer cells invasion. Both Egr-1 and HIF-1α were critical to angiogenesis induced by hypoxic conditions in MDA-MB-231 and HUVEC co-cultures. In nude mice, both Egr-1 and HIF-1α small interfering RNA (siRNA) could decrease extravasation of MDA-MB-435 cells in the lung after tail vein injection. Conclusions. Hypoxia-induced expression of TF in human breast cancer cells depends on Egr-1 and HIF-1α, and both of these proteins may play an important role in breast cancer metastasis, either directly or indirectly through the TF pathway.
Tissue factor (TF), a 47-kDa transmembrane protein, is frequently overexpressed in human tumors and functions in two manners, through induction of a signaling pathway and through its procoagulant activity. TF is no longer perceived only as a coagulation factor, but rather as a central trigger of the coagulation cascade as well as an important cell- associated signaling receptor. TF could be useful in guiding immune [Citation1] or pharmacological [Citation2] attacks on tumor microcirculation. The functional involvement of TF in tumor initiation, growth and angiogenesis renders it a logical target for the development of anti-tumor effects or anti-metastasis agents [Citation3]. Thus, recent promising research on TF suggests that this unique molecule could be explored in various ways to treat human cancer.
The TF promoter contains three overlapping early growth response gene-1 (Egr-1)/stimulating protein 1 (Sp1)-binding sites within a proximal enhancer region (2111 to +14 bp) [Citation4]. Egr-1 is a zinc finger transcription factor that belongs to a family of early growth response genes [Citation5]. This phosphoprotein rapidly accumulates in the nucleus upon stimulation by mitogens, a variety of cytokines, and cellular stress, including hypoxia [Citation6]. Egr-1 has been reported to regulate TF expression by displacing Sp1 from its binding site following external stimuli, whereas Sp1 is believed to mediate basal TF expression [Citation7]. Moreover, hypoxia-inducible factor-1α (HIF-1α) is highly expressed in breast cancer, especially under hypoxia [Citation8]. Hypoxia- responsive elements, which are the primary binding sites for HIF-1α, have not been reported within the TF promoter [Citation4]. Recent research has reported that hypoxia-mediated up-regulation of TF in glioblastoma multiforme cells depends largely on Egr-1 while being independent of HIF-1α. However, vascular endothelial growth factor (VEGF) up- regulates TF expression in endothelial cells through the activation of the VEGF receptor (VEGFR), which could suggest an indirect role of HIF-1α in the regulation of TF, as VEGF is a direct HIF-1α target gene [Citation9]. The mechanisms responsible for the increased expression of TF in hypoxic mammary cancer are currently not known. In the present study, we investigated the effects of Egr-1 and HIF-1α on the transcriptional regulation of TF expression in human breast cancer cells under hypoxia, as well as their potential for decreasing neoplastic metastasis.
Material and methods
Cell lines and cell culture
Human breast carcinoma MDA-MB-231 cells were cultured in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS, Gibco) and antibiotics (100 units/ml penicillin, and 100 mg/ml streptomycin). Human breast cancer MDA-MB-435 cells were maintained in DMEM with 10% FBS. Human umbilical vein endothelial cells (HUVEC) were obtained from umbilical veins and cultured as described previously [Citation8]. Endothelial cells were cultured in M199 medium (Sigma) with 20% FCS, supplemented with 50 ng/ml of endothelial cell growth supplement (Sigma) and 100 ng/ml heparin. Endothelial cells at the third to sixth passages were used. To create hypoxic conditions, 70–80% confluent cells were placed in an anaerobic chamber (HERAcell 150, Thermo) with 1% O2 and 5% CO2 at 37°C.
Adhesion assays
Adhesion assays were performed in 96-well culture plates. Briefly, triplicate wells were precoated overnight at 4°C with BSA (2% w/v, Sigma), fibronectin (10 μg/ml, Sigma), or vitronectin (10 μg/ml, Sigma). Cells were seeded at 2 × 104/well in serum-free DMEM or RPMI-1640 supplemented with 0.1% BSA. The cells pretreated with hypoxia or normoxia were allowed to adhere for 60 min at 37°C. Non-adherent cells were removed by gentle washing with phosphate buffered sodium (PBS), and adherent cells were measured with the CCK-8 kit (Dojindo Laboratories) [Citation10].
Invasion assays
Human breast cancer cell invasion was assayed in Transwell chambers (8 μm pore size; Corning, Lindfield, NSW, Australia). The surface of the filter membrane was coated with 30 μg of Matrigel (Sigma) for 2 h; 3 × 104 cells/well were seeded into the upper chamber and were allowed to invade toward a serum gradient (2% FBS) in the bottom well. After incubation at 37°C for 12 h under hypoxia or normoxia, migrated cells were stained and counted in five randomly selected fields as described previously [Citation8].
In vitro angiogenesis assays
The formation of capillary tube-like structures by HUVEC was analyzed on Matrigel. HUVEC were starved for 4 h in medium 199 with 1% FBS, seeded on the polymerized Matrigel (3 × 104cells/well). A co-culture model of HUVEC with MDA-MB-231 cells was established using Transwell chambers (0.4 μm pore size; Corning, Lindfield, NSW, Australia) in a 24-well format. Breast cancer cells were pretreated with Egr-1 or HIF-1α small interfering RNA (siRNA) seeded in the upper chambers, and then co-cultured with HUVEC seeded in the lower chamber and further incubated for 8 h in normoxia or hypoxia conditions [Citation11].
TF pro-coagulant activity determination
TF-activated factor VII (VIIa) compounds activate factor X into factor Xa which can break down a chromogenic substrate (chromogenic substrate, S-2222) into peptides and p-nitroaniline (pNA). Measurements of the latter at 405 nm can give an optical density (OD) value that reflects the level of TF pro-coagulant activity. Briefly, cell lysate (18 μl) and human prothrombin complex solution (2 μl, 0.01 g/ml, containing CaCl2 5 mM) were added into a 384-well plate and incubated at 37°C for 15 min. Subsequently, chromogenic substrate preheated to 37°C (20 μl, 0.5 mM, containing 25 mM ethylene diamine tetraacetic acid) was added to each well and incubated for 3 min, followed by measurement of the 405 nm absorbance value using a microplate reader [Citation12].
siRNA transfection
The chemically-modified oligonucleotides of siRNA for Egr-1-, HIF-1α- and Sp1-specific siRNA sequences were purchased from Ambion (Austin, TX; Genbank accession nos. NM_001964, NM_001530 and NM_138473). Transfections (50 nM Egr-1, HIF-1α or Sp1 siRNA) were accomplished using the Silencer siRNA Transfection II kit according to the supplier's instruction (Ambion). Twenty-four hours after transfection, MDA-MB-231, MDA-MB-435 cells were placed in hypoxia (1% O2) for 1 or 12 h, respectively.
Quantitative real-time PCR (qPCR)
qPCR was carried out on an iCycler Real-time PCR Detection System (Bio-Rad) using Sybr green PCR master mix (Applied Biosystems). The primiers used were: Egr-1 forward 5’-TGACCGCAGAGTCTT TTCCT-3’ and reverse 5’-TGGGTTGGTCATGC TCACTA-3’; TF forward 5’-GCCAGGAGAAA GGGGAAT-3’ and reverse 5’-CAGTGCAATAT AGCATTTGCAGTAGC-3’; HIF-1α forward 5’-CTCACCCAACGAAAAATTACAGAA-3’ and reverse 5’-ATTGAGTGCAGGGTCAGCACTAC-3’; β-actin forward 5’-TCACCCACACTGTGCC CATCTACGA-3’ and reverse 5’-CAGCGGAAC CGCTCATTGCCAATGG-3’. The amount of target gene mRNA relative to the internal control was calculated using the ΔΔCT method as follows: relative expression = 22ΔΔCT, ΔΔCT = ΔCT(test)2 ΔCT(calibrator).
Western blot
Western blot was performed according to our published method [Citation8]. Blots were probed with murine antibodies specific for human Egr-1 (R&D Systems), TF (R&D Systems), SP1 (Santa Cruz, CA), HIF-1a (Sigma), αv (Santa Cruz), β3 (Santa Cruz) and actin (Sigma). Horseradish peroxidase (HRP) linked anti-mouse immunoglobulin G (Sigma) was used as a secondary antibody. Immunoreactive proteins on the membrane were visualized by enhanced chemiluminescence (ECL) Western blotting detection reagents (Amersham, UK).
Enzyme-linked immunosorbent assay (ELISA)
Cells were incubated under hypoxic conditions for 12 h, and then conditioned medium was collected and stored at 280°C until analysis. MMP-2, MMP-9 and VEGF ELISAs were performed according to the manufacturer's instructions (R&D Systems).
Fluorescent labeling
Cells were incubated for 30 min at 37°C with serum-free Opti-MEM (Invitrogen) containing the fluorescent cell tracker dye rhodol-based fluorophore (CMRA, red fluorescent) or a 5-chloromethyl- fluorescein diacetate (CMFDA, green fluorescent) at 2 μM (Molecular Probes, Eugene/Oregon). After washing, cells were incubated for an additional 30 min with dye-free medium, washed, and trypsinized.
Tumor cell extravasation in nude mice
MDA-MB-435 cells infected with Egr-1 or HIF-1α siRNA were labeled in vitro with CMRA (red fluorescent) and normal MDA-MB-435 cells were labeled in vitro with CMFDA (green fluorescent) described earlier. Cells labeled with the red and green fluorescent dyes were mixed at a ratio of 1:1, and 2.5 × 106 cells suspended in PBS were introduced by tail vein injection. The ratio of green-to-red fluorescent cells in the injected suspension was measured by counting in a fluorescence microscope. Lungs were harvested 15 min or 7 h after injection. Lung tissues were sectioned in a cryostat and fluorescent cells in five random fields (200× magnification) of each section were counted [Citation13].
Statistical analysis
Quantitative data are expressed as means ± standard deviation (SD). Comparisons were analyzed by the Student's t-test. Significance was defined as p < 0.05.
Results
Role of Egr-1 in hypoxia-induced TF expression in breast cancer cells
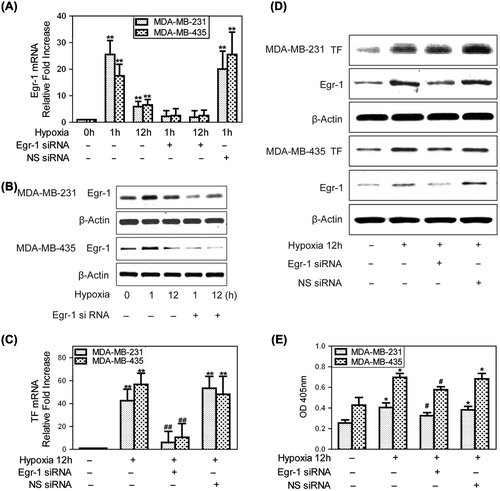
Hypoxia strongly induced Egr-1 mRNA levels in MBA-MB-231 and MDA-MB-435 cells within 1 h compared with normoxia. As shown in , Egr-1 mRNA levels were increased by 25.54 ± 5.24-fold in MDA-MB-231 and 17.50 ± 4.34-fold in MDA-MB-435 cells after hypoxia 1 h and by 5.93 ± 1.93- and 6.54 ± 2.06-fold, respectively, at 12 h. Similarly, increased Egr-1 protein levels were detected by Western blot (). Egr-1 siRNA markedly inhibited both Egr-1 mRNA (p < 0.01) and protein expressions under hypoxia. TF mRNA expression was strongly up-regulated by hypoxia (12 h) in both MDA-MB-231 and MDA-MB-435 cells and Egr-1 siRNA dramatically attenuated this hypoxia up-regulation of TF mRNA (). Similarly, hypoxia up-regulation of TF protein in both cell lines was significantly inhibited by Egr-1 siRNA (). Meanwhile, the activities of TF in MDA-MB-231 and MDA-MB-435 cells were increased under hypoxia. Egr-1 siRNA decreased this hypoxia-induced up-regulation of TF activity in both of the human breast cancer cells (). Together, these results showed that the increased Egr-1 expression occurring in hypoxic breast cancer cells can up-regulate TF expression.
Figure 1. Egr-1 is responsible for hypoxia-induced TF expression in human breast cancer cells. To create hypoxic conditions, cells were placed in an anaerobic chamber with 1% O2 and 5% CO2 at 37°C. (A) Egr-1 mRNA expression was analyzed by qRT-PCR and expression was recorded as the fold increase compared to that in normoxia. Values are mean ± SD for five experiments. **p < 0.01 vs. that of normoxia; (B) Western blot showing a marked increase in Egr-1 protein expression at both 1 and 12 h of hypoxia. Egr-1 siRNA significantly inhibited the hypoxia-mediated up-regulation of Egr-1 protein; (C) qRT-PCR for TF mRNA expression. Values are mean ± SD for five experiments. **p < 0.01 vs. that in normoxia; ##p < 0.01 vs. that in hypoxia; (D) The representative immunoblots show that hypoxia (12 h) strongly increased TF protein expression and this hypoxia-mediated up-regulation of TF protein was inhibited greatly by Egr-1 siRNA; (E) The activity of TF induced by hypoxia (12 h) was inhibited greatly by Egr-1 siRNA. Values are mean ± SD for five experiments. *p < 0.05 vs. that of normoxia; #p < 0.05 vs. that in hypoxia.
Sp1 is not up-regulated by hypoxia in breast cancer cells
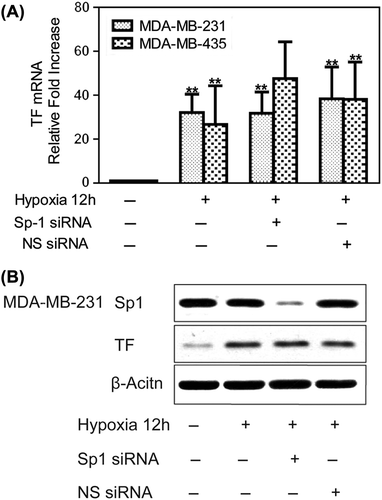
Since Sp1 is also a critical regulator of TF gene expression, we investigated whether it contributed to the increased expression of TF under hypoxia in human breast cancer cells. It was found that Sp1 siRNA in hypoxic MDA-MB-231 cells could significantly reduce the amount of Sp1 protein expression but did not substantially affect the hypoxia-induced TF expression (). Therefore, we concluded that Sp1 is not up-regulated by hypoxia in breast cancer cells and does not directly up-regulate TF under these conditions.
Figure 2. Hypoxia-mediated up-regulation of TF expression is independent on Sp1. To create hypoxic conditions, cells were placed in an anaerobic chamber with 1% O2 and 5% CO2 at 37°C. (A) MDA-MB-231 and MDA-MB-435 cells were transfected with Sp1 siRNA or non-specific siRNA the day before hypoxia treatment. Hypoxia (12 h) caused a large increase in TF mRNA expression, which was not affected by Sp1 siRNA treatment. Fold increase normalized to normoxia. Values are mean ± SD for five experiments. **p < 0.01 vs. that of normoxia; (B) Sp1 siRNA in MDA-MB-231 cells significantly inhibited Sp1 protein levels but did not affect the marked hypoxic up-regulation of TF.
HIF-1α and VEGF are also involved in hypoxia-mediated up-regulation of TF expression
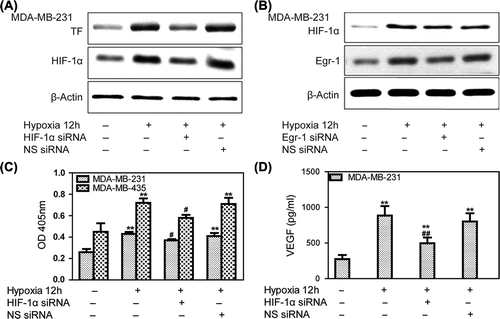
Silencing of HIF-1α by siRNA (50 nM) significantly inhibited its expression under hypoxia, whereas control MDA-MB-231 breast cancer cells showed strong up-regulation of HIF-1α. Meanwhile, HIF-1α siRNA inhibited hypoxia-mediated up-regulation of TF in MDA-MB-231 cells (). However, Egr-1 siRNA did not affect HIF-1α expression under hypoxia in MDA-MB-231 cells, indicating that the effects of Egr-1 on TF expression were not due to an indirect effect on HIF-1α (). HIF-1α siRNA decreased hypoxia-induced up-regulation of TF activity in MDA-MB-231 by 35.3% and in MDA-MB-435 cells by 51.9% (). Whereas hypoxia significantly increased VEGF levels in conditioned medium compared with that under normoxia (p < 0.05; ), it was significantly reduced in that of MDA-MB-231 cells treated with HIF-1α siRNA as determined by ELISA (p < 0.01). Thus, HIF-1α seemed to be a key but indirect regulator of the hypoxic expression of TF via the effects of VEGF.
Figure 3. Hypoxia-mediated up-regulation of TF expression is dependent on HIF-1α and VEGF. Human breast cancer cells were transfected with siRNA against HIF-1α during incubation in normoxia for 24 h. The cells were then incubated in 1% oxygen for 12 h before harvesting. (A) Western blots showing a marked increase in HIF-1α and TF protein expression at 12 h of hypoxia. TF protein expression could be attenuated by HIF-1α siRNA. (B) Western blots showing that Egr-1 siRNA did not affect HIF-1α expression. (C) Knockdown of HIF-1α decreased hypoxia-mediated up-regulation of TF activity in MDA-MB-231 and MDA-MB-435 cells. Values are mean ± SD for five experiments. **p < 0.01 vs. that in normoxia; #p < 0.05 vs. that in hypoxia; (D) HIF-1α siRNA attenuated hypoxia-induced VEGF secretion. Conditioned medium was collected, and VEGF was measured by ELISA. Values are mean ± SD for five experiments. **p < 0.01 vs. that in normoxia; ##p < 0.01 vs. that in hypoxia.
Effects of Egr-1 and HIF-1α on tumor metastases in vitro
To metastasize successfully, cells need to acquire a motile phenotype and to invade through the basement membrane and surrounding stroma. Site-specific metastasis has been proposed to be regulated, in part, by adhesion to and invasion towards the extracellular matrix (ECM) expressed at metastatic sites.
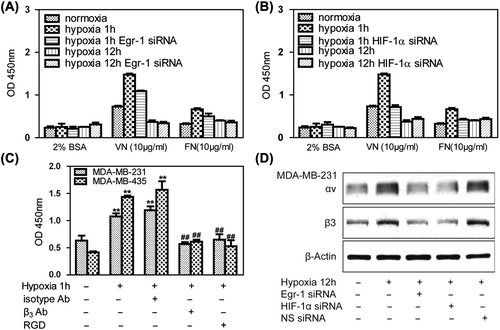
We first explored cell adhesion under hypoxic conditions. The MDA-MB-231 lines adhered only weakly to vitronectin and fibronectin in normoxia. Under hypoxia, however, adhesion of MDA-MB-231 cells to vitronectin was markedly increased, whereas only moderate binding to fibronectin was observed. Knockdown of Egr-1 () or HIF-1α () by RNA interference attenuated hypoxia-induced adhesion to cancer cells. The antagonist of αvβ3, polypeptide of RGD (Arg-Gly-Asp), and β3 antibody could attenuate hypoxia induced breast cancer cells adhesion to vitronectin, indicating that hypoxia-induced cell adhesion to vitronectin was mediated specifically via αvβ3 integrin (). Egr-1 siRNA and HIF-1α siRNA treated MDA-MB- 231 cells significantly inhibited αvβ3 protein levels, indicating that Egr-1/HIF-1α inhibited adhesion partially due to the αvβ3 pathway ().
Figure 4. Effects of Egr-1 and HIF-1α on tumor cell adhesion in vitro. To create hypoxic conditions, cells were placed in an anaerobic chamber with 1% O2 and 5% CO2 at 37°C. (A) MDA-MB-231 cells adhered weakly to vitronectin and fibronectin in normoxia. After 1 h of hypoxia, adhesion of MDA-MB-321 cells to vitronectin markedly increased. Egr-1 siRNA attenuated this hypoxia-induced adhesion of the cancer cells. (B) HIF-1α siRNA decreased the hypoxia-induced adhesion of breast cancer cells. (C) Treatment with a function-blocking anti-β3 integrin antibody and RGD (0.5 g/l) inhibited the adhesion of human breast cancer cells to vitronectin, indicating that this process is mediated specifically via αvβ3 integrin. (D) MDA-MB-231 cells were transfected with Egr-1 or HIF-1α siRNA 24 h before hypoxia treatment and analyzed for αvβ3 expression by Western blot. Egr-1 siRNA or HIF-1α siRNA affected αvβ3 expression.
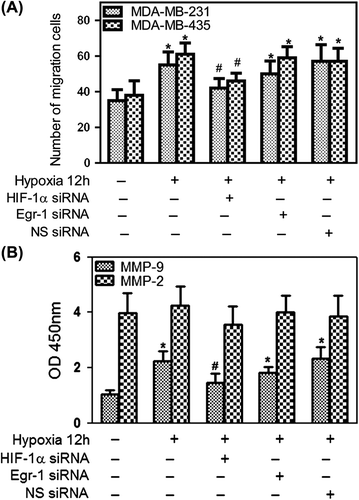
A transwell assay was performed while cells were cultured during hypoxia overnight to investigate the effects of hypoxia on cell invasion. As shown in , hypoxia significantly increased the invasive response of cancer cells (p < 0.05). Knockdown of HIF-1α by RNA interference reduced HIF-1α expression and attenuated hypoxia-induced invasion of cancer cells, while Egr-1 siRNA did not attenuate hypoxia-induced invasion of cancer cells (). Tumor cells are known to secrete MMPs, which are thought to degrade ECM and facilitate tumor cell invasion in tissues. As shown in , hypoxia resulted in an increase in MMP-9 secretion, while MMP-2 secretion was not changed. The effects of HIF-1α and Egr-1 on MMP-9 secretion by MDA-MB-231 cells were also examined. HIF-1α siRNA significantly attenuated the stimulation of MMP-9 secretion during hypoxia, while Egr-1 siRNA did not affect it. These results showed that HIF-1α contributed to MMP-9 secretion during hypoxia and augmented cell invasion in breast cancer cells, while Egr-1 decreased hypoxia-induced cells invasion, but it may be less important to this process.
Figure 5. Effects of Egr-1 and HIF-1α on tumor cell migration in vitro. To create hypoxic conditions, cells were placed in an anaerobic chamber with 1% O2 and 5% CO2 at 37°C. (A) Cell invasion was greatly increased under hypoxia. HIF-1α siRNA attenuated hypoxia-induced invasion of cancer cells, while Egr-1 just moderately decreased the cell invasion using 2% FBS as the chemoattractant. Average numbers of cells migrating in five randomly selected fields were counted 12 h after seeding. (B) Effects of Egr-1 and HIF-1α siRNA on MMP-2 or MMP-9 secretion. After blocking HIF-1α expression with 50 nM HIF-1α siRNA, MDA-MB-231 cells were cultured under hypoxic conditions in serum-free medium for 12 h; the conditioned media were collected and analyzed by ELISA. A decreased amount of MMP-9 was secreted in response to HIF-1α RNAi.
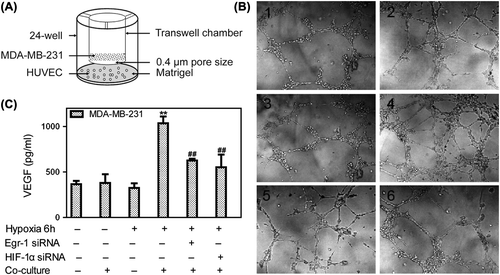
Co-culture with tumor cells under hypoxia conditions induced tubular network formation of endothelial cells plated on Matrigel (). Whether the cells were incubated in hypoxia or co-cultured with MDA-MB-231 cells in normoxia alone, the vascular tubule formation was not different from that under nomoxia. However, when the HUVECs were co-cultured with tumor cells under hypoxia, the capacity of endothelial cells to form tubules were enhanced significantly. MDA-MB-231 cells transfected by either Egr-1 siRNA or HIF-1α siRNA could inhibit HUVEC vascular tubule formation (). Hypoxia also caused a marked increase in the levels of VEGF in conditioned medium from the hypoxia co-culture compared with normoxia (p < 0.01), while knockdown Egr-1 or HIF-1α decreased those VEGF levels (). These results showed that Egr-1 and HIF-1α contributed to angiogenesis during hypoxia and co-culture HUVEC with breast cancer cells.
Figure 6. Effects of Egr-1 and HIF-1α on vascular tubule formation in vitro. To create hypoxic conditions, cells were placed in an anaerobic chamber with 1% O2 and 5% CO2 at 37°C. (A) HUVEC co-culture with MDA-MB-231 model was performed using transwell chambers adapted to 24-well format. The breast cancer cells were pretreated with Egr-1 or HIF-1α siRNA seeded in upper chambers, then co-cultured with HUVEC seeded in 24-well further incubated in normoxia or hypoxia conditions. (B) Vascular tubule formation was not different when HUVECs were incubated in hypoxia or in nomoxia or co-cultured with MDA-MB-231 cells in normoxia alone. When co-cultured with tumor cells in hypoxia, the capacity of endothelial cells to form tubules was enhanced significantly. Both Egr-1 siRNA and HIF-1α siRNA could inhibit vascular tubule formation (200× .1. Normoxia; 2. Normoxia + co-culture; 3. Hypoxia; 4. Hypoxia + co-culture; 5. Hypoxia + co-culture + Egr-1 siRNA; 6. Hypoxia + co-culture + HIF-1α siRNA). (C) Conditioned medium was collected from normoxic or hypoxic conditions of (B) and VEGF was measured by ELISA. VEGF levels in conditioned medium from hypoxia co-cultured cells higher than that from HUVEC cultured under normoxia. Values are mean ± SD for five experiments. **p < 0.01 vs. that in normoxia; ##p < 0.01 vs. that in hypoxia co-culture.
Egr-1 and HIF-1α decreases extravasation of MDA-MB-435 cells in vivo
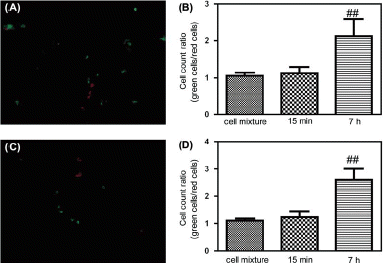
Cells were stained with the fluorescent dye CMRA or CMFDA, which are both cell permeable and, within cells, becomes entrapped by a covalent reaction with cytoplasmic proteins. The approach was used in nude mice to study MDA-MB-435 cell extravasation, with CMRA (red fluorescent) labeling of Egr-1 or HIF-1α siRNA treated MDA-MB-435 cells and CMFDA (green fluorescent) labeling of normal MDA-MB-435 cells. As shown in , MDA-MB-435 cells were observed in the lung at 7 h after tail vein injection, but less Egr-1 or HIF-1α siRNA treated cells than normal MDA-MB-435 cells were seen. In control studies, lungs harvested at 15 min showed relatively few fluorescent cells, mostly representing cells adherent to the lung endothelium that had not yet migrated. These data provide evidence for decreased lung extravasation of low-Egr-1-expressing MDA-MB-435 and low-HIF-1α-expressing MDA-MB-435 cells after tail vein injection.
Figure 7. Extravasation analysis of MDA-MB-435 cells in nude mice. (A) Fluorescence micrograph of frozen sections of a nude mouse lung at 7 h after tail vein injection of red CMRA-labeled Egr-1 siRNA treated MDA-MB-435 cells mixed 1:1 with green CMFDA-labeled normal MDA-MB-435 cells. (B) Tumor cell count ratios (green CMFDA-labeled normal MDA-MB-435 cells/red CMRA-labeled Egr-1 siRNA treated MDA-MB-435 cells) in lung at 15 min and 7 h after tail vein injection (n = 6, mean ± SD, **p < 0.01 compared with 1:1 cell mixtures). (C) Fluorescence micrograph of frozen sections of a nude mouse lung at 7 h after tail vein injection of red CMRA-labeled HIF-1α siRNA treated MDA-MB-435 cells mixed 1:1 with green CMFDA-labeled normal MDA-MB-435 cells. (D) Tumor cell count ratios (green CMFDA-labeled normal MDA-MB-435 cells/red CMRA-labeled HIF-1α siRNA treated MDA-MB-435 cells) in lung at 15 min and 7 h after tail vein injection (n = 6, mean ± SD, **p < 0.01 compared with 1:1 cell mixtures).
Discussion
The clinical importance of hypoxia for tumors is that it protects the cancer cells against radiation and chemotherapy, and it also regulates cell migration in inflammatory and neoplastic diseases by influencing leukocyte recruitment and activation, angiogenesis, and metastasis formation [Citation14]. HIF-1α is an important cytokine induced by hypoxia. It can influence tumor progress by activating its downstream proteins [Citation15,Citation16]. Hypoxia contributes to the expression and nuclear translocation of Egr-1[Citation17], which plays crucial roles in tumor invasion and metastasis [Citation18] and the tumor microenvironment [Citation19]. A growing body of evidence suggests that TF participates in cancer progression and metastasis [Citation20–22]. TF levels in mammary adenocarcinoma and other tumors correlate with increasing tumor progression, and an unfavorable prognosis mostly due to its prothrombotic and proangiogenic effects [Citation17]. The regulators of TF are diverse, such as Egr-1 [Citation23], the cooperative action of AP-1 and Egr-1 [Citation24], and MAPK/ERK [Citation25]. Mechanisms responsible for the increased expression of tissue factor in hypoxic mammary adenocarcinoma are not known. We explored the possible mechanisms of hypoxia-induced TF expression in mammary adenocarcinoma, especially the regulation effects of Egr-1/HIF-1α on TF. This study demonstrated for the first time that Egr-1 and HIF-1α are critical to hypoxia-mediated up-regulation of TF expression in human breast cancer cells. In addition, Egr-1 and HIF-1α are important determinants of breast cancer cells adhesion, invasion and angiogenesis and may be associated with mammary adenocarcinoma metastasis. The roles of Egr-1 and HIF-1α in tumor metastasis may be direct or indirect through the TF pathway.
Our studies have shown that the hypoxic induction of TF expression in human breast cancer depends on the up-regulation of Egr-1, a transcriptional regulator known to be rapidly induced by several microenvironmental stimulants, including hypoxia, growth factors, and hormones. Previous studies have shown that Egr-1 is the major mediator of TF expression in epithelial cells [Citation6], mononuclear phagocytes [Citation6], and endothelial cells [Citation26]. However, the role of Egr-1 in the hypoxia-induced expression of TF in mammary tumors has not been explored fully. Sp1 and Egr-1 are zinc finger transcription factors that share three GC-rich binding regions within the TF promoter that are critical for maintaining its basal activity [Citation27]. We have shown that the proximal Egr-1/Sp1-binding sites in the TF promoter are also necessary for its hypoxia-induced activity in human breast cancer cells. Sp1 was constitutively expressed in breast cancer cells and showed no increased expression under hypoxia. Hypoxia-induced TF expression could be attenuated by Egr-1 siRNA, whereas these findings were not observed by using Sp1 siRNA. Together, our results indicate that Egr-1 is the most critical element engaging the Egr-1/Sp1-binding site of the TF promoter in hypoxic human breast cancer cells and that Sp1 likely plays a role in the basal expression of TF.
In endothelial cells, the regulation of TF has been shown to depend on VEGF and requires the activation of the VEGFR [Citation8]. Because the VEGF gene is under the transcriptional regulation of HIF-1α, which is strongly up-regulated in breast cancer, the expression of TF could be under the indirect regulation of HIF-1α in breast cancer cells. We found that HIF-1α was responsible for the hypoxic up- regulation of TF expression through VEGF. Thus, HIF-1α is certainly critical for the hypoxic induction of angiogenesis that occurs in breast cancer, and it may be regulating this process through the expression of TF under hypoxia.
Up-regulated TF expression by tumor cells may be critical for the development of tumor growth, metastasis process and, angiogenesis [Citation28]. Since Egr-1 and HIF-1α are critical for the hypoxic induction of TF expression in breast cancer, it is rational to suppose that Egr-1 and HIF-1α may play important roles in tumor metastasis. By use of in vitro and in vivo assays, we showed that both Egr-1 and HIF-1α are critical to hypoxic induction of breast cancer cell metastasis.
The integrin family has been shown to play a critical role in cell proliferation, migration, invasion and colonization, as well as promoting cell survival [Citation10,Citation29]. Our observation is consistent with these previous studies. Egr-1 or HIF-1α siRNA could decrease cancer cells adhesion to vitronectin and inhibit the expression of αvβ3. We used the matrigel invasion assay to determine the effect of Egr-1 or HIF-1α on mammary carcinoma cell lines and found that cell invasion was greatly increased under hypoxia. HIF-1α siRNA attenuated hypoxia-induced invasion of cancer cells and decreased MMP-9 levels up-regulated by hypoxia. However, Egr-1 may play a less important role than HIF-1α in cancer cell invasion. These data implicated the pivotal role of Egr-1 and HIF-1α in cancer cell adhesion and the critical role of HIF-1α in cancer cells invasion during hypoxia.
The growth of solid tumors is dependent on angiogenesis, as a tumor requires ample blood supply for expansive growth. When HUVECs were incubated in hypoxia or co-cultured with MDA-MB-231 cells in normoxia alone, vascular tubule formation was not different from that of nomoxia, but the capacity of endothelial cells to form tubules was enhanced significantly when the HUVECs were co-cultured with tumor cells under hypoxia. Combined with the VEGF ELISA assay, we concluded that VEGF up-regulated by hypoxia in MDA-MB-231 cells enhanced HUVEC tubule formation. Thus, both Egr-1 and HIF-1α played important roles in angiogenesis in the co-culture system under hypoxia.
Metastasis is the overwhelming cause of mortality in patients with breast cancer, and our understanding of its cellular and molecular determinants is limited. TF is the principal physiologic initiator of coagulation. It also plays an important role in tumor growth and metastasis possibly by contributing to adhesion, invasion and angiogenesis [Citation30,Citation31]. Our study has provided evidence that both Egr-1 and HIF-1α are critical to the hypoxic up-regulation of TF expression in breast carcinoma cells. Notably, these factors may play important roles in breast cancer metastasis and may represent significant therapeutic targets that should be considered in the future treatment of breast cancer metastasis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was financially supported by The anti-metastatic research of Saponin Monomer of Dwarf Lilyturf Tuber (DT-13): (NO.81102853).
References
- Hu ZW, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci USA 2001;98:12180–5.
- Huang X, Molema G, King S. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997;275:547–50.
- Versteeg HH, Schaffner F, Kerver M. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008;111: 190–9.
- Mackman N. Gene targeting in hemostasis: Tissue factor. Front Biosci 2001;6:208–15.
- Rong Y, Hu F, Huang RP, Mackman N, Horowitz JM, Jensen RL, et al. Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1 – independent mechanisms. Cancer Res 2006;66:7067–74.
- Yan SF, Zou YS, Gao Y. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA 1998;95: 8298–303.
- Cui MZ, Parry GC, Oeth P. Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and Egr-1. J Biol Chem 1996;271:2731–9.
- Lin SS, Sun L, Hu JL, et al. Chemokine C–X–C motif receptor 6 contributes to cell migration during hypoxia. Cancer Lett 2009;279:108–17.
- Shen BQ, Lee DY, Cortopassi KM, Damico LA, Zioncheck TF. Vascular endothelial growth factor KDR receptor signaling potentiates tumor necrosis factor-induced tissue factor expression in endothelial cells. J Biol Chem 2001;276: 5281–6.
- Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, et al. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res 2006;8:R20–34.
- Guo K, Li J, Wang HH, Osato M, Tang JP, Quah SY, et al. PRL-3 initiates tumor angiogenesis by recruiting endothelial cells in vitro and in vivo. Cancer Res 2006;66:9625–35.
- Miura M, Seki N, Koike T, Ishihara T, Niimi T, Hirayama F, et al. Design, synthesis and biological activity of selective and orally available TF/FVIIa complex inhibitors containing non-amidine P1 ligands. Bioorg Med Chem 2007; 15:160–73.
- Yong J. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life 2009; 61:1001–9.
- Semenza L. HIF-1 and tumor progression: Pathophysiology G and therapeutics. Trends Mol Med 2002;8:S62–7.
- Haque I, Banerjee S, Mehta S, De A, Majumder M, Mayo MS, et al. Cysteine-rich 61-connective tissue growth factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1- induced signaling protein-2 (WISP-2) regulates microRNA-10b via hypoxia-inducible factor-1α-TWIST signaling networks in human breast cancer cells. J Biol Chem 2011; 286:43475–85.
- Du J, Xu R, Hu Z, Tian Y, Zhu Y, Gu L, et al. PI3K and ERK-induced Rac1 activation mediates hypoxia-induced HIF-1α expression in MCF-7 breast cancer cells. PLoS One 2011;6:e25213.
- Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 2005; 65:1406–13.
- Shimoyamada H, Yazawa T, Sato H, Okudela K, Ishii J, Sakaeda M, et al. Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am J Pathol 2010;177:70–83.
- Shin SY, Kim JH, Baker A, Lim Y, Lee YH. Transcription factor Egr-1 is essential for maximal matrix metalloproteinase-9 transcription by tumor necrosis factor alpha. Mol Cancer Res 2010;8:507–19.
- Schaffner F, Yokota N, Ruf W. PL-27 Tissue factor proangiogenic signaling in cancer progression. Thromb Res 2012; 129:S127–31.
- de Oliveira AD, Lima LG, Mariano-Oliveira A, Machado DE, Nasciutti LE, Andersen JF, et al. Inhibition of tissue factor by ixolaris reduces primary tumor growth and experimental metastasis in a murine model of melanoma. Thromb Res Epub 2012 Jun 9.
- Ma Z, Zhang T, Wang R, Cheng Z, Xu H, Li W, et al. Tissue factor-factor VIIa complex induces epithelial ovarian cancer cell invasion and metastasis through a monocytes-dependent mechanism. Int J Gynecol Cancer 2011;21:616–24.
- Rong Y, Hu F, Huang R, Mackman N, Horowitz JM, Jensen RL, et al. Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1-independent mechanisms. Cancer Res 2006;66:7067–74.
- Kim J, Min JK, Park JA, Doh HJ, Choi YS, Rho J, et al. Receptor activator of nuclear factor kappaB ligand is a novel inducer of tissue factor in macrophages. Circ Res 2010; 107:871–6.
- Hu C, Huang L, Gest C, Xi X, Janin A, Soria C, et al. Opposite regulation by PI3K/Akt and MAPK/ERK pathways of tissue factor expression, cell-associated procoagulant activity and invasiveness in MDA-MB-231 cells. J Hematol Oncol 2012;5:16.
- Mechtcheriakova D, Wlachos A, Holzmuller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood 1999;93:3811–23.
- Sarraj A, Day RM, Thiel G. Specificity of transcriptional regulation by the zinc finger factors Sp1, Sp3, and Egr-1. J Cell Biochem 2005;94:153–67.
- Rak J, Milsom C, Magnus N, Yu J. Tissue factor in tumor progression. Best Pract Res Clin Haematol 2009;22: 71–83.
- White DE, Muller WJ. Multifaceted roles of integrins in breast cancer. J Mammary Gland Biol Neoplasia 2007;12:135–42.
- Zhao W, Wang YS, Hui YN, Zhu J, Zhang P, Li X, et al. Inhibition of proliferation, migration and tube formation of choroidal microvascular endothelial cells by targeting HIF-1 alpha with short hairpin RNA-expressing plasmid DNA in human RPE cells in a coculture system. Graefes Arch Clin Exp Ophthalmol 2008;246:1413–22.
- Forstera Y, Meyeb Al, Albrecht S, Kotzsch M, Füssel S, Wirth MP, et al. Tissue specific expression and serum levels of human tissue factor in patients with urological cancer. Cancer Lett 2003;193:65–73.
