Abstract
Background. The Late Effects Normal Tissue/Subjective Objective Management Analytic (LENT/SOMA) system for grading of side effects after radiotherapy was proposed several years ago. Only a few studies have previously been performed on the validity of the LENT/SOMA. The aim of the present study was to validate the LENT/SOMA scoring system for recto-anal side effects after treatment for prostate cancer in a randomized trial. Material and methods. A total of 875 patients with locally advanced prostate cancer were randomized to either hormonal treatment alone or hormonal treatment plus radiotherapy in the Scandinavian Prostate Cancer Group 7 (SPCG-7) study. At least three years after treatment was started, the 178 patients that were randomized at St. Olavs Hospital were approached. One hundred and three patients of these accepted inclusion. The side effects according to LENT/SOMA were graded by oncologist and nurse. In addition, side effects were graded according to the European Organisation for Research and Treatment of Cancer and the Radiation Therapy Oncology Group (EORTC/RTOG) toxicity scale and patient-reported health-related quality of life (HRQOL) questionnaires. Content/face validity, sensitivity and inter-rater reliability of the LENT/SOMA tables for rectum were analyzed. Results. Content/face analysis of LENT/SOMA revealed serious problems. Significant correlations (Spearman's rho > 0.4) were found between three of 15 LENT/SOMA items and similar HRQOL items. LENT/SOMA score made it possible to detect significant differences between the two groups of patients (p < 0.001), EORTC/RTOG toxicity score did not (p = 0.138). Inter-rater reliability was acceptable. Conclusions. LENT/SOMA scoring system for recto-anal side effects after radiotherapy for prostate cancer displays serious difficulties in the present study. Replacement of LENT/SOMA tables for rectum by a combination of patient-reported HRQOL questionnaires, clinical examination and objective physiological measurements might be called for.
Late side effects on normal tissue from curative radiotherapy (RT) have gained increased scientific and clinical interest over the last decades. A wide range of evaluation methods for late side effects have been used in the literature, from patient-reported symptom scores via professional care provider reported scoring systems to objective, physiological measurements such as recto-anal manometry. Professional healthcare providers have reported late side effects by using the European Organisation for Research and Treatment of Cancer and the Radiation Therapy Oncology Group (EORTC/RTOG) toxicity grading scale in a number of studies [Citation1–3].
The Scandinavian Prostate Cancer Group 7/Swedish Society for Urological Oncology 3 (SPCG-7) study was initiated in 1995 and concluded in 2002. The object of the SPCG-7 study was to evaluate the effect of RT plus HT versus HT alone on survival in patients with locally advanced prostate cancer in a prospective, randomized design. The results of the trial reported reduction in mortality when adding RT to HT in locally advanced prostate cancer [Citation4].
Radiotherapy for prostate cancer can induce several types of gastrointestinal morbidities such as proctitis and recto-anal dysfunction. There are multiple reports on recto-anal side effects after pelvic radiotherapy [Citation5], including a variety of evaluation methods. Thus, to compare results between studies and to conduct a meta-analysis is difficult.
The LENT/SOMA tables for grading of RT-induced side effects were developed in 1995 by an expert working group within the EORTC/RTOG. The group used the EORTC/RTOG toxicity scoring system as a basis, with the purpose to improve it and develop a system which ‘embodies simplicity of design and will result in accuracy of detail’ [Citation6]. Since then, the LENT/SOMA scoring system for the rectum has been applied in reports on radiotherapy for prostate cancer [Citation5,Citation7,Citation8], although validation reports remain relatively few [Citation5,Citation6,Citation9–12]. Validation if the LENT/SOMA tables for non-pelvic malignancies have also been reported, demonstrating that LENT/SOMA was the most accurate scale when compared to RTOG and National Cancer Institute – common toxicity criteria (NCI-CTC) [Citation13]. The LENT/SOMA tables have later been included as part of the Common toxicity criteria for adverse events (CTCAE version 3.0) which has been recommended as common platform for reporting of adverse events [Citation14].
The aim of the present study was to analyze the content/face validity, sensitivity and inter-rater (nurse vs. doctor) reliability of the LENT/SOMA scoring system for radiation-induced recto-anal toxicity.
Material and methods
Patients
The SPCG-7 study included prostate cancer patients stages T1G3, T2G2-G3, T3G1-3 N0M0. Patients were randomized to HT alone (three months total androgen blockage, TAB, followed by antiandrogen) or HT plus RT. Details of the study population and treatment are previously reported [Citation4,Citation15].
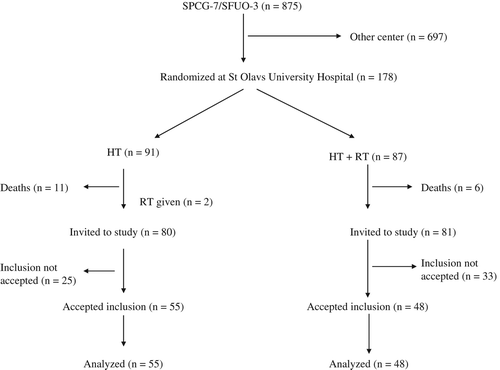
A total of 178 patients were included by randomization in the SPCG-7 study at St. Olavs Hospital (). Seventeen patients died prior to start of the present study, leaving 161 patients to be invited. These patients were approached at least three years after randomization for inclusion in the present study. The invitation was sent by mail, accompanied by HRQOL questionnaires EORTC QLQ-C30 [Citation16] and QUFW-94 [Citation17].
Fifty eight patients declined and 103 patients accepted the invitation and were included in the present study. Of these, 55 patients were randomized to HT alone, whilst 48 were randomized to HT plus RT.
Questionnaires
The EORTC QLQ-C30 version 3.0 consists of 30 single items (questions), where patients are asked to grade their symptoms on a 10-point numerical rating scale. These items cover six functional scales that measure physical, role, cognitive, social and emotional function, as well as global quality of life (QOL).
The QUFW-94 is a prostate cancer specific patient-reported questionnaire that consists of 39 single items, mainly scored in a 0–10 numerical rating scale. The intestinal part of this questionnaire contains 13 questions. Three of these are dichotomous or descriptive. With one exception, the rest of the single items are scored in such a manner that the value ‘0’ represents ‘no symptoms’ and ‘10’ means very severe symptoms. The single item on stool consistency is also scored from 0–10, but for this item, ‘0’ means ‘very loose’ and ‘10’ represents ‘very hard’. A combination of EORTC QLQ-C30 and the QUFW-94 was used to report HRQOL in the SPCG-7 study and in the present study.
The EORTC/RTOG score for side effects from radiotherapy is a multiple item scoring system resulting in one of six grades where ‘0’ implies no toxicity whereas ‘5’ implies side effect related death. The scoring is performed by healthcare professionals, based upon clinical evaluation of the patients.
The LENT/SOMA score is a multiple item scoring system composed of four domains; subjective, objective, management and analytic. The scoring is performed by healthcare professionals, based upon an interview and clinical examination.
Completion of questionnaires
Patients were asked to complete these questionnaires after the clinical visit. Reasons for not accepting inclusion were not investigated, as recommended by the regional ethical committee. Upon attendance and after giving written consent, all patients were clinically interviewed and examined including rectoscopy by first author who completed the LENT/SOMA and EORTC/RTOG scoring forms. The LENT/SOMA objective domain was graded by means of rectoscopy. After having observed mucosal changes in several patients, the mucosal findings at rectoscopy were graded and included as the analytic domain [Citation18]. Furthermore, one of four trained nurses also conducted a clinical interview in order to fill in the LENT/SOMA grading. Prior to the start of the study, the participating nurses were trained in how to use the LENT/SOMA grading system. The nurses’ grading was performed only for those items not including a clinical examination, i.e. excluding the objective domain items regarding bleeding, ulceration and stricture and the ‘Analytic’ domain.
Strategy for analyses
Evaluation of the EORTC/RTOG and the LENT/SOMA was performed with five different approaches:
1. The content/face validity was analyzed through a systematic evaluation of the content and grading of each item within the two measures.
2. Content comparison of the LENT/SOMA and the HRQOL questionnaires (EORTC QLQ-C30 and QUFW-94) was performed to identify single items in LENT/SOMA similar to single symptom items in EORTC QLQ-C30 and QUFW-94. Based on the content of these single items, an attempt was made by the authors to rate the items with regards to relevance between LENT/SOMA and QLQ-C30 plus QUFW-94. The following criteria were applied: When the LENT/SOMA item and the HRQOL item seemed to fully cover the same content the content relevance was given a rate of 1. When the LENT/SOMA item content seemed to partially cover the HRQOL item content or vice versa, a rating of 2 was applied.
3. Based on the content comparison, correlation analyses between LENT/SOMA versus QLQ-C30 and QUFW-94 were performed. Correlations were computed between single item scores from LENT/SOMA with relevant single item scores in QLQ-C30 and QUFW-94 by non-parametric correlations (Spearman's rho). Based on the assumption that a large percentage of these patients would have a LENT/SOMA score of 0, one would expect low correlation coefficients. Hence, evidence of correlation was set at Spearman's rho ≥ 0.4.
4. The sensitivity of the LENT/SOMA tables in detecting recto-anal RT-induced toxicity was analyzed by comparing the total LENT/SOMA grading performed by oncologist and the EORTC/RTOG grading by treatment arms. Differences between treatment arms were evaluated by the non-parametric Mann Whitney U-test for both the LENT/SOMA score and the EORTC/RTOG score. All sensitivity analyses were performed by intention to treat.
5. Inter-rater reliability was evaluated by difference in total maximum score done by physician versus nurse. The difference between raters was tested by weighted Kappa (quadrate of difference). The objective and analytic domains were omitted in the inter-rater testing.
Data handling was performed using SPSS version 15.0 (SPSS Inc, Chicago, Illinois, USA). The study was approved by the Regional Ethical Committee.
Results
Baseline characteristics
Two patients randomized to HT alone were given RT due to local progression during the time from randomization in the SPCG-7 study to time of inclusion in the present study. Three patients received 70 Gy to both the prostate and the seminal vesicles, 46 patients received 50 Gy to the seminal vesicles and 70 Gy to the prostate, and one patient received 50 Gy to the seminal vesicles and 76 Gy to the prostate. Patients in the two treatment arms were similar with regards to baseline information ().
Table I. Baseline characteristics for 103 patients diagnosed with prostate cancer treated by HT*+ RT† versus HT alone in a randomized trial.
Compliance to questionnaires and scoring systems
All included patients were graded according to both EORTC/RTOG and LENT/SOMA scores by the oncologist, and all included patients were graded according to LENT/SOMA score by the nurse. The oncologist completed at least 99% of all items in the subjective and management domains, while in the objective domain, at least 85% of the items were completed. Grading of mucosal changes was achieved in 54 patients (52%).
The nurse completed at least 99% of all included items.
The compliance for the QLQ-C30/QUFW-94 single items identified by the content analyses amongst the included patients varied from 90% to 93%.
Face/content validity of EORTC/RTOG toxicity score
The EORTC/RTOG toxicity score is composed of 10 single items (); one item on pain, four items related to bowel/rectum pathophysiology (stools, mucus and blood), four items on local rectal pathophsiology (necrosis, perforation, obstruction, fistula) and the final item is death. The radiotherapy toxicity is graded on a six-point scale ranging from 0 to 5; however the grading system varies between items. Four items are graded 0 to 2, one item 0 to 3, while five items are dichotomous and graded as 0 or 4. The last item, death, is also dichotomous but graded as 0 or 5. Four of the five items having a multiple grading are also described with verbal statements of various grading levels. It is not indicated what type of pain that is to be measured, however one may assume that it is meant to measure pain intensity. Pain grading stops at grade 2 – ‘moderate colic’ – and a similar grading is used for stool consistency which also stops at grade 2 verbalized as ‘moderate’. However, grade 2 for mucus is anchored with ‘severe’. The grading for blood relies on different verbal anchors; grades 0 and 1 are related to the amount of blood, grade 2 is related to frequency while grade 3 is related to the need of an intervention.
Table II. Content of the European Organisation for Research and Treatment of Cancer and the Radiation Therapy Oncology Group (EORTC/RTOG) radiotherapy toxicity scoring system.
Face/content validity of LENT/SOMA
The subjective domain is composed of five items, three of which are also included in the EORTC/RTOG scale (mucosa, stool frequency and pain) (). Two new items have been added; sphincter control and tenesmus. Most of these items are found in several domains; tenesmus, frequency, pain and sphincter control are all included both in the subjective and the management domains. The objective domain is composed of three items, of which one item on bleeding is included in the EORTC/RTOG scale. The two remaining items (ulceration and stricture) have been added from the original EORTC/RTOG score. All of these three items are also included in the management domain. The management domain is constructed of five items that hence also are included in the subjective or objective domains.
Table III. Contents of the Subjective Objective Management/Late Effects Normal Tissue (SOMA/LENT) tables for rectum.
The grading of the LENT/SOMA is based on a five-point scale, ranging from 0 to 4. All items include all grades, and for all items grade 0 is verbally described as ‘no toxicity’. For three of the items in the subjective domain (tenesmus, mucosal loss and sphincter control), grades 1–3 relates to frequency of the symptoms – verbalized as occasional, intermittent and persistent, respectively. Grade 4 is for these items described as ‘refractory’ which is a verbal descriptor that include intensity, frequency and response to treatment. The fourth subjective item, stool frequency, is graded from 1–3 based on the number of stools per day, while grade 4 is verbally anchored as ‘uncontrolled diarrhea’. The last subjective item, pain, is described with verbal statements that cover both pain intensity and frequency for grades 1–3, while grade 4 is phrased in order to include intensity, frequency and response to treatment (‘refractory’).
The objective domain's item bleeding is graded by means of various verbal descriptors. Grade 1 is defined as ‘occult’, grades 2 and 3 are phrased to cover the patient's reporting of frequency and persistence, while grade 4 is related to the volume of blood per rectum. The grading of the objective item ulceration is defined by verbal statements that relate to the depth and the area of the ulceration for grades 1 and 2, while for grade 3 ‘deep’ is applied as the verbal descriptor. Grade 4 is verbally defined by ‘perforation or fistula’. The final objective item, stricture, is graded by various verbal descriptors pointing to the narrowness of the rectal lumen. The differences between grades 1, 2 and 3 are based on the clinicians’ evaluation of how small the rectal lumen is, with dilatation. Grade 4 is defined as ‘complete obstruction’.
The management domain item tenesmus and frequency grades 1–3 are defined by verbal descriptors of the frequency of intake of medication to stop diarrhea. Grade 4 is defined as ‘surgical intervention/permanent colostomy’. The item on pain grades 1–3 are verbally anchored in statements describing the frequency of intake and the type of pain medication being used. Grade 4 is defined as ‘surgical intervention’. Management items bleeding, ulceration and stricture are graded according to various statements regarding possible actions being taken by the patient. Stool softener and iron intake define grade 1 toxicity. Grades 2 and 3 are stated as the need for medical intervention (transfusion, steroid medication, dilatation), and for items on bleeding and stricture, the frequency of the intervention separates grade 2 from grade 3 toxicity (occasional vs. frequent or occasional vs. regular). The difference between grades 2 and 3 for single item ulceration is verbalized as difference in the frequency and specificity of medical interventions (occasional steroids vs. steroids per enema, hyperbaric oxygen). The final item in the management domain, sphincter control, is differentiated in grades by statements regarding the frequency of use of incontinence pads for grades 1–3, while grade 4 is phrased as ‘surgical intervention/permanent colostomy’.
Translation
The Norwegian and the original English version of LENT/SOMA differ: Single items subjective mucosal loss, sphincter control and management sphincter control have been removed in the Norwegian version. In addition, the grading of single item subjective pain has been simplified; the original version apply both intensity and frequency of pain as criteria for grades 1–3, while the Norwegian version apply frequency alone. For grade 4, both versions include both frequency and intensity as criteria. It was not possible to identify any formal or informal translation procedures [Citation8].
Content comparison LENT/SOMA versus patient-reported questionnaires
The content comparison made by the authors between LENT/SOMA and the HRQOL package displayed that seven LENT/SOMA items were found to have similar content as five EORTC QLQ-C30/QUFW-94 items (). LENT/SOMA and QLQ-C30/QUFW-94 single item association achieved a rating of 1 (fully identical content) for two comparisons: LENT/SOMA Subjective Frequency and QUFW-94 question ‘How many stools in 24 hours do you have?’ and LENT/SOMA Subjective tenesmus and QUFW-94 question ‘Did you have cramp/pain when passing stools?’ The other relevant content comparisons were given a rating of 2 (partially identical content) by the authors.
Table IV. Correlation coefficients (Spearman’s rho) for SOMA/LENT † vs QlQ-C30/QUFW-94†† single items in 103 patients randomiized to hormonal treatment alone or hormonal treatment + radiotherapy for prostate cancer.
Correlation analyses LENT/SOMA versus patient-reported questionnaires
Spearman's rho for QLQ-C30/QUFW-94 versus LENT/SOMA single items are presented in . Three correlations reached the pre-determined correlation level.
Sensitivity analyses LENT/SOMA and EORTC/RTOG.
LENT/SOMA score (all items) and EORTC/RTOG score by oncologist according to treatment is presented in . The difference between treatment groups was statistically significant for the LENT/SOMA score (p < 0.001) but not statistically significant for the EORTC/RTOG score (p = 0.138).
Table V. SOMA/LENT* and EORTC/RTOG† toxicity score for rectum in 103 patients randomized to hormonal therapy (HT) alone versus HT plus radiotherapy (RT).
Inter-rater reliability.
Inter-rater reliability is presented in . Eight patients (7.7%) differed by two grades or more when comparing the oncologist's score to the nurse's score. The absolute value of the difference between the nurse's score and the oncologist's score resulted in the following: In 71 patients, the difference was 0, in 24 patients the difference was one, six patients differed by two grades, one patient by three and one patient by four grades. For the patient with a difference of three grades between raters, there was a difference in the registered tenesma, i.e. the nurse did not register any tenesma while the oncologist registered tenesma grade 3. For the patient with a difference of four grades, the difference was found in the single subjective item on pain, the doctor registered no pain while the nurse registered pain grade 4. Both care providers registered grade 0 on the single management item on pain medication for this patient. The weighted Kappa calculation showed similar toxicity scores by groups of care providers (estimated kappa = 0.52, p < 0.001).
Table VI. SOMA/LENT* score for rectum performed by doctor versus nurse in 103 patients treated for prostate cancer in a randomized trial comparing HT † versus HT + RT ††.
Discussion
Based upon the results of the face/content validity analyses the EORTC/RTOG toxicity score revealed serious content problems. First of all, the scoring algorithm of EORTC/RTOG results in a total score equal to the single item having the highest score. This implies that a patient with mild to moderate symptoms from several single items would be graded with ‘lower toxicity’ as compared to a patient having, for instance a rectal obstruction that could be handled surgically by dilatation, rendering a patient free from symptoms. Furthermore, only one symptom can result in grade 3 toxicity, i.e. bleeding. This could in most studies result in under-reporting of grade 3 toxicity.
Also, the grading regarding frequency of stools is limited in that precisely five stools per 24 hours would result in grade 1 toxicity, whereas more than five would result in grade 2 and less than five would result in grade 0. This scoring represent a bias in that very few patients will be given a grade 1 toxicity score based on stool frequency. Furthermore, it would seem reasonable to divide the patients having more than five stools per day into several intervals based on stool frequency.
Finally, it does not seem reasonable that a patient cannot be scored to a grade 3 or 4 based on symptoms such as mucus, increased frequency or pain. It is a matter of deep concern that the EORTC/RTOG toxicity score has been applied in modern reports on late normal tissue toxicity [Citation1,Citation3].
The LENT/SOMA form was possible to apply on almost all included patients, as indicated by the excellent compliance by both doctor and nurse for the single items. The lowest percentage of scoring achieved was in the objective and the analytic domains.
The intentions of the researchers constructing the LENT/SOMA tables are well-formulated, and should be acknowledged. However, the process preceding the construction of the LENT/SOMA tables is poorly described in the literature, and the ideas behind the different grading are not clear [Citation6,Citation9,Citation10].Furthermore, the lack of validity studies on the LENT/SOMA tables for rectum is striking compared to the intentions of validation studies made by the authors of the LENT/SOMA tables. The fact that clinicians and clinical researchers do not have standardized, validated instruments available for measuring late side effects from RT is a major problem. Compared to the instruments applied in, for instance clinical chemistry laboratories, the tools for clinical measurements seem far more disputable. It is also a major problem that there is no broad international consensus on which instrument to use so instead researchers have developed their own instruments [Citation19] making meta-analysis almost impossible to perform. We therefore support the authors of the QUANTEC studies in their attempt to standardize measures of RT toxicity [Citation20].
The content analyses of the LENT/SOMA tables for rectum revealed a number of difficulties: The subjective domain is based on the patient's response to the healthcare provider even though most researchers believe that subjective symptoms are best assessed by the patients themselves [Citation21]. Grading of the single item subjective pain is also difficult, in that the original version of the LENT/SOMA tables include intensity, frequency and patients’ coping of pain as pain measurements. This problem could not be explored further in the present data set, due to the Norwegian translation of the LENT/SOMA tables. However, a 0–10 numerical rating scale for pain is by most researchers considered a more precise measurement of pain and should probably be implied [Citation22].
The content of the items in each domain of the LENT/SOMA tables is not always logically related to the grading of the domain. For instance, the objective bleeding item is based on a laboratory test for grade 1 (occult bleeding). For grades 2 and 3, however, the score is based on medical history from the patient regarding frequency and severity of rectal bleeding, hence being a part of a subjective domain. Furthermore, the objective stricture and ulceration items are graded based on the examiner's subjective evaluation of the stricture or ulceration, and one might anticipate high inter-rater variability for these items. In the present study, the inter-rater reliability for the objective domain could not be analyzed due to study design.
The authors’ grading of content association between LENT/SOMA and QLQ C-30/QUFW-94 must be considered a subjective analysis. The difficulties of this analysis mainly consisted of the subjectivity involved when evaluating the degree of contents similarity between two similar questions. It is still worth noting that only two of the LENT/SOMA items were given a content association rating 1 when comparing them to single items in QLQ-C30 and QUFW-94. This implies that the contents of the single items of the LENT/SOMA tables are very different from the contents of the single items included in two validated well-established patient-reported outcome measures. This finding was re-enforced by the correlation analyses of the same single items, displaying low correlation coefficients for all but three single items (stool frequency, bleeding and pain). Even though toxicity measures and patient-reported quality of life measures measure different concepts, we believe that the content analyses presented here indicate that the LENT/SOMA single items not necessarily measure what they were designed to measure.
The Norwegian translation [Citation8] was reduced in content compared to the original presentation of the LENT/SOMA tables [Citation10]. The report by Bakken et al. did not describe the methodology applied when translating the LENT/SOMA tables. Such translation should always be performed according to guidelines, in order to avoid introducing errors during the translation process [Citation23]. One cannot expect that clinicians applying such translated questionnaires or scoring tables will take the time to compare the translated version with the original version.
The LENT/SOMA score's sensitivity to RT- induced toxicity was clearly better than the EORTC/RTOG score's sensitivity in detecting RT-related side effects. If these patients had been evaluated merely by the EORTC/RTOG toxicity score, the results would indicate that patients randomized to RT + HT did not have any higher recto-anal toxicity than patients randomized to HT alone, which escalates the concerns regarding the EORTC/RTOG toxicity score.
This study indicates that measuring of late RT-induced recto-anal toxicity needs to be improved. The EORTC/RTOG toxicity score should not be used in future trials. Furthermore, the observations presented here calls for a major revision of the LENT/SOMA or even may be a formal development of a new toxicity score based upon systematic literature review, experimental patient studies and systematic validation procedures. We support the strategy of combining patient- and clinician-reported outcomes [Citation24,Citation25] to achieve a common platform for measuring RT toxicity.
Declaration of interest: The author Widmark (Sanofi-Aventis) has received lecture fees less than EUR 2000. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol 2007;17:131–40.
- Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Long-term outcomes. Int J Radiat Oncol Biol Phys 2005;63:1463–8.
- Bolla M, Van Poppel H, Collette L, Van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911). Lancet 2005;366:572–8.
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 2009;373:301–8.
- Livsey JE, Routledge J, Burns M, Swindell R, Davidson SE, Cowan RA, et al. Scoring of treatment-related late effects in prostate cancer. Radiother Oncol 2002;65:109–21.
- Rubin P, Constine LS, Fajardo LF, Phillips TL, Wasserman TH. RTOG Late Effects Working Group. Overview. Late Effects of Normal Tissues (LENT) scoring system. Int J Radiat Oncol Biol Phys 1995;31:1041–2.
- Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74.
- Bakken AM, Ogreid P, Iversen J, Langberg CW. [Normal tissue radiation toxicity in radiotherapy of localized prostatic cancer]. TidsskrNor Laegeforen 1999;119:2017–21.
- Denekamp J, Bartelink H, Rubin P. Correction for the use of the SOMA LENT tables. Int J Radiat Oncol Biol Phys 1996;35:417.
- Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. EORTC Late Effects Working Group. Late effects toxicity scoring: The SOMA scale. Radiother Oncol 1995;35:11–5.
- Anacak Y, Yalman D, Ozsaran Z, Haydaroglu A. Late radiation effects to the rectum and bladder in gynecologic cancer patients: The comparison of LENT/SOMA and RTOG/EORTC late-effects scoring systems. Int J Radiat Oncol Biol Phys 2001;50:1107–12.
- Lundby L, Laurberg S, Overgaard J. Is the LENT-SOMA scoring system useful to record late rectal toxicity?RadiotherOncol 2000;57(Suppl 1):S16.
- Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: Comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys 2003;55:93–8.
- Davidson SE, Trotti A, Ataman OU, Seong J, Lau FN, da Motta NW, et al. Improving the capture of adverse event data in clinical trials: The role of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2007;69:1218–21.
- Fransson P, Lund JA, Damber JE, Klepp O, Wiklund F, Fossa S, et al. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol 2009;10:370–80.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality- of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Widmark A, Fransson P, Tavelin B. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer 1994;74:2520–32.
- Feldman M, Friedman Lawrence S, Sleisinger Marvin H. Sleisinger & Fordtran’s gastrintestinal an liver disease; pathophysiology, diagnosis, management. Philadelphia: Saunders; 2003. p. 2048.
- Lilleby W, Fossa SD, Waehre HR, Olsen DR. Long-term morbidity and quality of life in patients with localized prostate cancer undergoing definitive radiotherapy or radical prostatectomy. Int J Radiat Oncol Biol Phys 1999;43:735–43.
- Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, et al. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S155–60.
- Ganz PA, Gotay CC. Use of patient-reported outcomes in phase III cancer treatment trials: Lessons learned and future directions. J Clin Oncol 2007;25:5063–9.
- Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: New information from multidimensional scaling. Pain 1996;67:267–73.
- Koller M, Aaronson NK, Blazeby J, Bottomley A, Dewolf L, Fayers P, et al. Translation procedures for standardised quality of life questionnaires: The European Organisation for Research and Treatment of Cancer (EORTC) approach. Eur J Cancer 2007;43:1810–20.
- Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 2011;103:1808–10.
- Farnell DJ, Mandall P, Anandadas C, Routledge J, Burns MP, Logue JP, et al. Development of a patient- reported questionnaire for collecting toxicity data following prostate brachytherapy. Radiother Oncol 2010;97:136–42.