To the Editor,
Lung tumours move during breathing, and tumour motion of more than 3 cm has been seen for tumours located near the diaphragmatic domes [Citation1]. Breathing-adapted radiotherapy such as respiratory beam gating or tumour tracking, rely on the ability to determine and predict the breathing-related tumour motion based on an external or internal surrogate for tumour motion [Citation2]. The prediction of the correlation between the tumour and the surrogate positions must be verified throughout the treatment; the verification can be performed with repeated kV-imaging of the tumour. However, not all lung tumours are well-defined on kV-images and therefore radio-opaque markers implanted in or close to the tumour have been used as a surrogate for tumour position. Markers can be implanted percutaneously guided by fluoroscopy or computed tomography (CT) or trans-bronchially inserted in nearby small bronchi. The advantage of percutaneous implantation is the possibility to implant the marker directly into the tumour assuring a good representation of tumour motion, but potentially at the cost of morbidity due to the risk of pneumothorax. We report the results of a small pilot study examining the feasibility of CT-guided percutaneous implantation of fiducial markers.
Material and methods
Fifteen patients were prospectively included between February 2009 and December 2010. Data collection was approved by the Danish Data Protection Agency (j.nr. 30-0484). The study was approved by the local ethical committee (j.nr. H-B-2007-016) and reported to Clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00910546).
Study inclusion criteria were: referral for SBRT, non-small cell lung cancer or lung metastases, age > 40 years, WHO performance status (PS) < 2 and obtained signed informed consent. Exclusion criteria were: forced expiratory volume in one second (FEV1) < 0.5 l, centrally located tumour, or tumour located close to large vessels.
All marker implantations were performed CT-guided by the same radiologist one week prior to treatment planning and two weeks prior to treatment start. Markers and implantation devices were changed during the study. For the first five patients a VisicoilTM (IBA Dosimetry, Bartlett, TN, USA) gold marker measuring 0.75 mm × 2 cm was implanted with an 18 gauge needle (outer diameter 1.27 mm). Patient no. 6 had a complex helical platinum coil (Boston Scientific, Natick, MA, USA) measuring 20 mm restrained, 2 × 4× 4 mm unrestrained, implanted using an 18 gauge needle. Patients no. 7–15 had a Gold AnchorTM marker (Naslund Medical, Huddinge, Sweden) measuring 0.28 × 20 mm implanted using a 25 gauge needle (outer diameter 0.53 mm). An improved inner coating of the preloaded needle was later introduced and used for patients no. 11–15. In all patients, only a single marker was implanted. All markers were complex in structure ().
Figure 1. Photo showing the complex helical platinum marker (top), the Gold AnchorTM marker (middle) and the VisicoilTM gold marker (bottom).

After implantation a thoracic CT was acquired to check for pneumothorax. The patients were observed for four hours followed by a chest x-ray. In case of pneumothorax, a thoracic surgeon was consulted regarding further intervention and/or observation.
The planning protocol for stereotactic body radiation therapy (SBRT) consisted of a positron emission tomography (PET)/CT with contrast enhancement and, within two subsequent days, a four-dimensional (4D)CT and a voluntary deep inspiration breathhold CT (BHCT) in the same session. Treatment planning was performed on the midventilation phase of the 4DCT and a total dose of 45 Gy was delivered in three fractions within 5–8 days.
Results
Patient characteristics are presented in . The marker of patient no. 2 disappeared between the planning scan and the treatment start. It had been placed in proximity to a bronchus and the patient had been reported coughing. The marker was probably expectorated, as a full-body CT revealed no sign of the marker.
Table I. Patient characteristics.
Patients no. 4, 5, 7 and 10 experienced a pneumothorax. None of the patients had symptoms of pneumothorax (lowering of blood oxygen saturation, increased breathing rate, dyspnoea or cough). Nevertheless three of the patients had a more than 3 cm collapse of the lung on x-ray and required a chest tube. For patients no. 7 and 10 the marker was initially not properly detached and withdrawn with the needle. A new marker was placed within the same session. The collapsed lung of patient no. 4 had not fully unfolded at the planning session and the patient had to be readmitted for a second chest tube thereby delaying treatment for a week. Hospitalisation time was 1–6 days (median 2.5 days). Patient no. 3 had haemoptysis before and after the marker implantation. In patient no. 6, the only patient with the complex helical platinum coil, the marker was implanted more than 1 cm below the tumour ().
Figure 2. Chest x-rays showing different marker types. Patient 1 had a 2 cm long helical gold marker (VisicoilTM) implanted in the tumour in the right inferior lung lobe (left). Patient 6 had the complex helical platinum marker (Boston Scientific) implanted in the tumour in right superior lung lobe (middle). Note that the marker was placed outside the tumour. The patient had a pacemaker. Patient 11 had the Gold AnchorTM marker implanted in the tumour in the right inferior lobe (right). The arrows indicate the position of the markers.
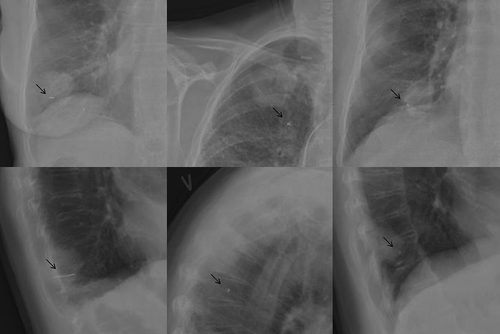
By visual evaluation of the thoracic orthogonal kV x-rays acquired four hours after the marker implantation (), it was clear that the Visicoil marker provided the highest radio-opacity. The two other markers provided equal radio-opacity. However, the radio-opacity of the Gold AnchorTM marker depended on the intra-tumoral folding of the marker.
Discussion
In our study four of 15 patients (2/5 with Visicoil and 2/9 with Gold anchor) developed pneumothorax after CT-guided percutaneously marker implantation. In the two patients that developed pneumothorax after the 25 gauge needle implantation, the implantation was performed twice, since the marker was initially withdrawn with the needle, indicating that implantation of more markers may increase the risk of pneumothorax. A larger ratio of patients developed pneumothorax when implantation was done using the larger 18 gauge needle than with the thinner 25 gauge needle although not significantly in this small sample size.
The pneumothorax rate in this study did not exceed what previous studies using percutaneous implantation have reported [Citation3–11], except for the study by de May et al. [Citation8] where a pneumothorax rate of only 10% was reported. Indeed in a study by Kupelian et al. [Citation12], also including 15 patients for percutaneous implantation of VisicoilTM markers, eight of the patients had pneumothorax. The high pneumothorax rate requires that the benefit of marker implantation is substantial in terms of increased precision in treatment delivery and decreased toxicity to normal tissue with breathing-adapted radiotherapy. Planning studies have shown potential clinical benefit of breathing-adapted radiotherapy. However advanced breathing adaptation, e.g. respiratory gating or tumour tracking without markers and extensive image guidance, is difficult [Citation13]. The real clinical benefit of breathing-adapted (marker guided) radiotherapy needs yet to be confirmed in large trials.
A limitation of the present study is the limited number of patients included and the long inclusion period. Although the radiologist implanting the markers was trained in interventional procedures and had performed hundreds of CT-guided biopsies, a learning curve can be expected when performing a new procedure, as he was not familiar with marker implantation prior to this present study. Thus the complication rate may be overestimated in this small study. The use of different markers represented an unscheduled modification of the clinical protocol. The gold VisicoilTM marker gave the best radio-opacity in kV-imaging but the implantation needle was an 18 gauge needle and resulted in two cases of pneumothorax after just five implantations, why the use was omitted. The next marker, the complex helical platinum marker, was not preferred by our radiologist due to manual loading into a needle, in contrast to the two other types of markers that were preloaded. Last we used the Gold AnchorTM marker which was inserted through a thin 25 gauge needle minimising the risk of pneumothorax. The thin needle was very flexible making the steering of the needle challenging. In our opinion the optimal marker for IGRT and insertion method need yet to be found.
Overall, we conclude that percutaneous implantation of markers was feasible but associated with a significant risk of pneumothorax. Larger clinical trials with continuous implantation of markers for breathing adaptation are warranted to estimate a reliable complication rate and to further improve breathing-adapted radiotherapy of lung cancer.
Acknowledgements
The authors have received grants from the Danish Council for Independent Research in Medical Sciences, The Arvid Nilssons Foundation, The Astrid Thaysens Foundation and the Lundbeck Foundation Center for Interventional Research in Radiation Oncology (CIRRO). The authors wish to thank Ingemar Näslund for providing the Gold AnchorTM implantation devices.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874–900.
- Taylor ML, Kron T, Franich RD. A contemporary review of stereotactic radiotherapy: Inherent dosimetric complexities and the potential for detriment. Acta Oncol 2011;50: 483–508.
- Yousefi S, Collins BT, Reichner CA, Anderson ED, Jamis-Dow C, Gagnon G, et al. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer 2007;8:252–6.
- Sotiropoulou E, Stathochristopoulou I, Stathopoulos K, Verigos K, Salvaras N, Thanos L. CT-guided fiducial placement for cyberknife stereotactic radiosurgery: An initial experience. Cardiovasc Intervent Radiol 2010;33:586–9.
- Pennathur A, Luketich JD, Heron DE, Schuchert MJ, Burton S, Abbas G, et al. Stereotactic radiosurgery for the treatment of lung neoplasm: Experience in 100 consecutive patients. Ann Thorac Surg 2009;88:1594–600.
- Nuyttens JJ, Prevost JB, Praag J, Hoogeman M, Van Klaveren RJ, Levendag PC, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol 2006; 45:961–5.
- Kothary N, Heit JJ, Louie JD, Kuo WT, Loo BW, Jr., Koong A, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol 2009;20:235–9.
- de Mey J, Van de Steene J, Vandenbroucke F, Verellen D, Trappeniers L, Meysman M, et al. Percutaneous placement of marking coils before stereotactic radiation therapy of malignant lung lesions. J Vasc Interv Radiol 2005;16:51–6.
- Collins BT, Erickson K, Reichner CA, Collins SP, Gagnon GJ, Dieterich S, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol 2007;2:39.
- Collins BT, Vahdat S, Erickson K, Collins SP, Suy S, Yu X, et al. Radical cyberknife radiosurgery with tumor tracking: An effective treatment for inoperable small peripheral stage I non-small cell lung cancer. J Hematol Oncol 2009;2:1.
- Bhagat N, Fidelman N, Durack JC, Collins J, Gordon RL, LaBerge JM, et al. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc Intervent Radiol 2010;33:1186–91.
- Kupelian PA, Forbes A, Willoughby TR, Wallace K, Manon RR, Meeks SL, et al. Implantation and stability of metallic fiducials within pulmonary lesions. Int J Radiat Oncol Biol Phys 2007;69:777–85.
- Korreman SS, Juhler-Nottrup T, Fredberg PG, Navrsted PA, Enmark M, Nystrom H, et al. The role of image guidance in respiratory gated radiotherapy. Acta Oncol 2008;47: 1390–6.
