Abstract
During the past decade planning of adjuvant radiotherapy (RT) of early breast cancer has changed from two-dimensional (2D) to 3D conformal techniques. In the planning computerised tomography (CT) scan both the targets for RT and the organs at risk (OARs) are visualised, enabling an increased focus on target dose coverage and homogeneity with only minimal dose to the OARs. To ensure uniform RT in the national prospective trials of the Danish Breast Cancer Cooperative Group (DBCG), a national consensus for the delineation of clinical target volumes (CTVs) and OARs was required. Material and methods. A CT scan of a breast cancer patient after surgical breast conservation and axillary lymph node (LN) dissection was used for delineation. During multiple dummy-runs seven experienced radiation oncologists contoured all CTVs and OARs of interest in adjuvant breast RT. Two meetings were held in the DBCG Radiotherapy Committee to discuss the contouring and to approve a final consensus. The Dice similarity coefficient (DSC) was used to evaluate the delineation agreement before and after the consensus. Results. The consensus delineations of CTVs and OARs are available online and a table is presented with a contouring description of the individual volumes. The consensus provides recommendations for target delineation in a standard patient both in case of breast conservation or mastectomy. Before the consensus, the average value of the DSC was modest for most volumes, but high for the breast CTV and the heart. After the consensus, the DSC increased for all volumes. Conclusion. The DBCG has provided the first national guidelines and a contouring atlas of CTVs and OARs definition for RT of early breast cancer. The DSC is a useful tool in quantifying the effect of the introduction of guidelines indicating improved inter-delineator agreement. This consensus will be used by the DBCG in our prospective trials.
The first national guidelines for adjuvant radiotherapy (RT) of breast cancer in Denmark were made by the Danish Breast Cancer Cooperative Group (DBCG) in 1977. Until 2006 the majority of patients referred for postoperative RT were treated using two- or three-field techniques planned on a simulator guided by external markers and bony landmarks. Typically the treatment technique after breast conserving surgery or mastectomy included an anterior field against the lymph nodes (LN) in the clavico-axillary region in combination with tangential opposed fields to the breast region. In case of postmastectomy irradiation an electron field was often used to the chest wall [Citation1]. Patients treated with this technique had a significantly lower loco-regional recurrence (LRR) rate and improved overall survival compared to non-irradiated patients [Citation2–4]. Since 2003 a gradual change in treatment planning from two-dimensional (2D) to computed tomography (CT)-based 3D was made in Denmark, thus the DBCG Radiotherapy Committee in 2006 revised the guidelines for postmastectomy RT to include treatment based on a CT scan [Citation5]. Shortly after, CT-based planning after breast conserving surgery was also introduced and since 2007 3D planning has been the national standard for all patients treated with adjuvant breast RT in Denmark.
The purpose of introducing CT-based target delineation was to optimise clinical target volumes (CTV) dose coverage and homogeneity and minimise organs at risk (OAR) dose. Since data from patients treated during the period with 2D planning has demonstrated a very low risk of LRR after adjuvant RT, it was important to assure that no significant change in the field arrangements and/or sizes of the fields during the shift from 2D to 3D was introduced. Other groups have proposed institutional guidelines for target definition in adjuvant breast cancer RT [Citation6–10], but to our knowledge no national guidelines have been presented. In the DBCG Radiotherapy Committee we monitor the LRR rates in all patients treated with adjuvant RT and we initiate prospective national RT protocols. It is therefore important to reach a national consensus on target delineation in order to minimise contouring uncertainty and maximise a uniform treatment planning. The aim of the present study was to provide the first Danish consensus on delineation of CTVs and OARs in adjuvant breast RT to be used for patients treated according to the DBCG guidelines.
Material and methods
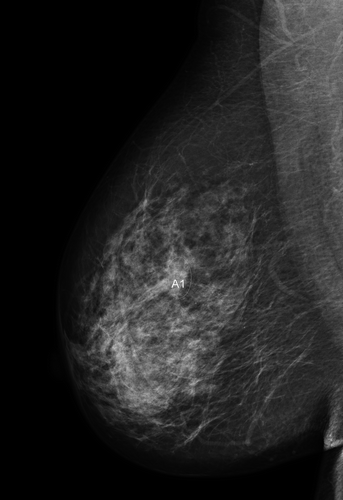
A series of national workshops with the participation of radiation oncologists and medical physicists experienced in adjuvant breast RT from each of the eight Danish RT centres was held between 2010 and 2012. During multiple dummy-runs CTVs and OARs delineation was performed by seven radiation oncologists on a contrast enhanced planning CT scan of a 62-year-old woman operated for a screening-detected right-sided breast cancer with microscopically radical breast conservation and level I–II axillary clearance. The mammography () identified a tumour at 1 o´clock 6 cm from the papillary complex. The tumour bed was marked intra-operatively with four metallic clips. Pathological tumour size was 13 mm, invasive ductal carcinoma, malignancy grade 1, 100% eostrogen receptor positive, HER2 negative, and there were two macrometastases among the 14 axillary LN removed. The patient was positioned supine in treatment position with both arms symmetrically abducted approximately 120˚ in a breast board CivcoTM (Medtec) and with the sternal bone in horizontal position. The palpable breast was demarcated with a radiopaque wire. Another wire was placed cranio-caudally in the mid-sternal line and one on the surgical scar. Intravenous contrast was administered immediately prior to scanning. The scan was acquired during free breathing, and covered the area from the mid-neck to the upper abdomen with a slice thickness of 2 mm. Delineation of anatomical structures was performed by experienced oncologists (one from each seven Danish RT centre and one representing two centres) using either the Eclipse™ (Varian Medical Systems Inc.), Oncentra External Beam™ (Nucletron, An Elekta Company) or Pinnacle™ (Philips) treatment planning systems. The following CTVs were delineated: breast, tumour bed, axillary LN (level I, II and III), the periclavicular LN (medial supraclavicular, lateral supraclavicular and infraclavicular), the internal mammary LN and the interpectoral LN. The following OARs were delineated: heart, left anterior descending coronary artery (LADCA), ipsilateral lung, larynx and spinal cord. Delineations of the lung and spinal cord were performed by an automatic contouring function, and corrected manually if needed.
Figure 1. The mammography identified a tumour at 1 o´clock 6 cm from the papillary complex in the right breast.
Delineation was performed prior to the workshops to facilitate the discussion process to reach the final consensus.
During the meetings guidelines for target delineation in patients operated with mastectomy were also discussed, so we provide our guidelines for delineation of the chest wall too.
The resulting national guidelines and atlas were approved by the DBCG Radiotherapy Committee. Only the delineation of CTVs in a standard patient will be described in this article, since the definition of the planning target volume depends on institutional guidelines.
The same oncologists delineated CTVs and OARs according to the consensus guidelines, approximately six months after the national delineation consensus was approved.
Delineation similarities before and after the consensus among the individual delineation were evaluated using the Dice similarity coefficient (DSC). DSC is defined as the intersection volume between volume A and (a reference) volume B divided by the mean of volumes of A and B [Citation11,Citation12]. Hence the coefficient is confined to the interval [0;1] and a DSC close to 0 or 1 indicates no or perfect overlap with the reference structure, respectively. The consensus delineated CTVs and OARs all served as reference volumes when calculating the DSC. The DSC was not calculated for the LADCA (often the LADCA cannot be visualised on a planning CT scan due to motion of the heart), lung or spinal cord (since the latter are contoured automatically).
Results
Preconsensus delineation
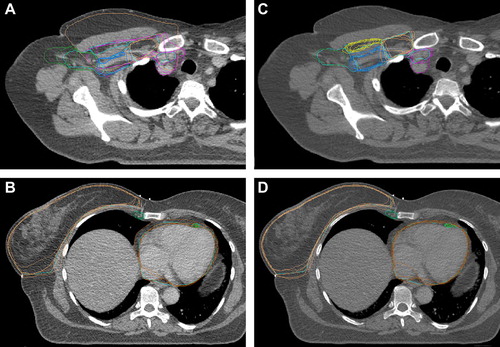
During the first dummy-run all participating centres submitted a set of delineated breast and lymph node targets as well as risk volumes. A representative example of the delineations performed is shown in and B. It documents clinically relevant differences in the delineated volumes.Thus, a national consensus was needed.
Figure 2. Representative examples of preconsensus and postconsensus delineations. A and B: (to the left) Preconsensus delineations. C and D: (to the right) Postconsensus delineations. CTV-breast: orange, CTV axillary LN level I: bright green, CTV axillary LN level II: blue, CTV axillary LN level III: orange, CTV interpectoral LN: yellow, CTV periclavicular LN: pink, CTV IMN: forest green.
Consensus delineation
shows representative examples of CTVs and OARs from the consensus delineation. The full CT atlas with all consensus delineations is available online (http://www.dbcg.dk : Retningslinjer/Vejledninger). The guidelines for delineation describing the anatomical boundaries for each CTV and OAR both after breast conserving operation and mastectomy are shown in .
Figure 3. Representative examples of CTVs from the consensus delineation. The full CT atlas is available online (http://www.dbcg.dk : Retningslinjer/Vejledninger). CTV- breast: orange, Tumour bed: red, CTV axillary level I: bright green, CTV axillary level II: blue, CTV axillary level III: orange, CTV interpectoral LN: yellow, CTV IMN: forest green, CTV periclavicular LN: pink, Ipsilateral lung: green.
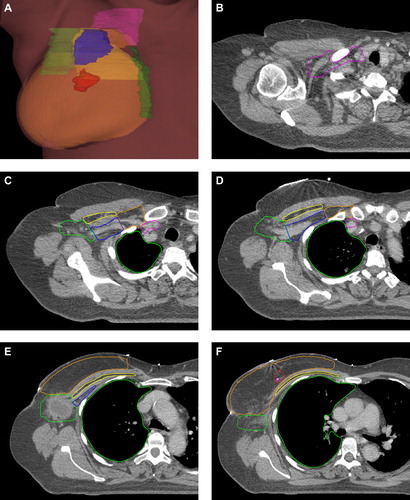
Table I. The national guidelines for delineation of target volumes in adjuvant radiotherapy of early breast cancer.
Constraints used by DBCG
During dose planning we strive to achieve a dose distribution in the CTV breast or CTV chest wall of 95–107% (normofractionated RT) according to the ICRU criteria [Citation13]. For RT of lymph nodes we accept a dose distribution within the range of 90–107%. If the dose inhomogeneity is higher than 107% it is recommended to lower the total dose to avoid double trouble. It has high priority to spare the OAR as much as possible from radiation, and our constraints are shown in . The priorities are:
Table II. Constraints for organs at risk in adjuvant radiotherapy of early breast cancer.
CTV-boost > LADCA > heart > lung > CTV-breast/chest wall > CTV-periclavicularis > CTV-IMN > contralateral breast.
Postconsensus delineation
During a second dummy-run six months after the approval of the national delineation guidelines a new delineation set was submitted by all eight centres. A representative example of the delineations performed postconsensus is demonstrated in and . It shows a higher degree of contouring agreement among the RT centres. Averages and ranges of the DSC are presented in for the relevant regions of interest (ROIs). For all ROIs the DSC improved after consensus. It was proposed by Zijdenbos et al. that a coefficient larger than 0.70 represents a good overlap [Citation14]. This was accomplished by the average DSC for only two of eight ROIs before but for seven of nine ROIs after consensus.
Table III. Mean and ranges of DSC before and after consensus.
Discussion
Postoperative loco-regional RT in patients with early breast cancer has shown a significant benefit in lowering the risk of loco-regional failures and death from breast cancer both after breast conserving surgery and mastectomy [Citation2–4,Citation15,Citation16]. This fundamental knowledge was acquired during the era of simple 2D planned RT. Today's CT planned 3D conformal RT enables the precise volumetric and geographical definition of CTV as well as OAR. Deijkema et al. [Citation8] were pioneers in describing the anatomical borders of the different loco-regional lymph node areas in planning CT scans, however, using these guidelines we observed an unwarranted increase in the irradiated volumes compared to the volumes used in the days of 2D planning.
Breast, tumour bed and chest wall
In the postlumpectomy situation the CTV breast includes all mammary glandular tissue. The lateral border of the breast may sometimes be difficult to define in a planning scan of patients with gland involution. However, in most cases it is possible even in a scan without intravenous contrast to identify the small axillary vessels running in cranio-caudal direction. These vessels (the lateral thoracorsal vessel with the lateral mammary branches) may be used as a surrogate marker of the lateral border of the breast. Gland involution may also dim the cranial and medial borders of the breast; the inferior edge of the clavicle and the lateral edge of the sternum can be used as surrogate markers of the cranial and medial borders of the breast, respectively.
In the DBCG consensus the CTV boost is defined as the tumour bed expanded by 5 mm in all directions without exceeding the CTV breast. The tumour bed includes all surgically inserted clips and the solid tissue and/or seroma in-between. All relevant information from the preoperative mammography, ultrasound and in some cases MR mammography together with the description of the surgical procedure is used in the delineation of the tumour bed. During breast conserving surgery the Danish surgeons insert 4–6 clips in the lumpectomy cavity. In case of oncoplastic surgery the clips are inserted in the cavity before transposition of the breast tissue so the radiotherapist has to be aware of possible movement of the clips if a boost is planned. For mastectomy the borders of the CTV chest wall are guided by the position of the contra-lateral breast.
In case of bilateral mastectomy the caudal border is defined as 3 cm caudal of the mastectomy scar. The borders and the mastectomy scar are marked with radiopaque wires before the planning CT scan. The ventral border is 5 mm below the skin surface, except for a region 3 cm cranially and caudally to the mastectomy scar where a bolus of 3–6 mm thickness is applied to ensure adequate dose in the skin. Sometimes the mastectomy scar extends outside the breast region to achieve a good cosmetic result, however, only the part of the mastectomy scar inside the breast region is target as we do not consider tumour contamination to be a clinical problem. The dorsal border of the chest wall is the ventral side of the major pectoral muscle; if this is difficult to define the ribs can be used instead.
Regional LN
The exact border between two lymph node areas is in most cases not sharp. However, using more advanced field techniques such as intensity modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) it is pivotal to avoid gaps between adjacent LN volumes to ensure homogenous dose in the intersection. Compared to the guidelines from other institutions [Citation8,Citation10] our consensus guideline for the interpectoral LN CTV results in a quite large volume [Citation10]. Our rationale is to ensure treatment of the upper medial quadrant of the chest wall, particularly in the postmastectomy situation, as was the case during the era of 2D planning (to avoid a ‘cold spot’ in the dose planning).
In contrast to other institutional guidelines the DBCG consensus proposes to limit the posterior border of axillary LN to 5 mm dorsal to the axillary vessels. When using the CTV definitions by Dijkema [Citation8] we observed that the irradiated volume increased compared to 2D planning. In our experience based on the 2D planning the risk of a regional recurrence in and posterior to level II after previous regional irradiation is very small (unpublished observations). Since part of the brachial plexus is located just posterior to the axillary vessels we find it important to be restrictive with the dose to this region to avoid later nerve morbidity.
In cases with severe lymph node involvement we include level I in the RT fields. The definition of ‘severe involvement'is not exact. It is based on the decision of the clinician, however at least 50% of the removed LN should be tumour positive. The tradition in the 2D era was to limit the RT fields around the edge of the humeral head, and using 3D scans this corresponds to defining the cranial border of level I around 1 cm caudal to the humeral head to avoid later arm morbidity. Since the risk of recurrence in the posterior part of level I in our experience is very low, we exclude the most posterior part of this lymph node region positioned between the chest wall and the dorsal muscles.
In the days of 2D planning the periclavicular region was defined by a cranial border corresponding to the lower edge of the sixth cervical vertebra and a lateral border corresponding to two thirds of the length of the clavicle. Regarding the medial border most centres used the position of a wire following the anterior edge of the sternocledo-mastoideus muscle to spare the thyroid gland, eosophagus and larynx from a high dose. Based on this routine we recommend the exclusion of the most medial part of the periclavicular LN from the CTV. Caudally the periclavicular LNs meet the IMN behind the sternum and are here located so dorsally that this region may be excluded from the CTV to avoid excessive lung irradiation, taking in consideration that a large percentage of the patients have been treated with chemotherapy just prior to the RT and therefore have a larger risk of side effect.
OARs and constraints
The delineation of the heart and left anterior descending coronary artery (LADCA) is based on the guidelines from Feng [Citation17] and is defined by the heart muscle including the complete pericardia from the lower part of the pulmonic trunc to the apex. By including the complete pericardia we insure that all coronary arteries are within the heart outline. LADCA is delineated from aorta including the left main artery to the cardiac apex cordis. It is often difficult to visualise the caudal part of the LADCA but here a surrogate is the interventricular gap.
Dice similarity coefficient (DSC)
As discussed earlier the DSC can readily be used as a measure of the overlap for larger volumes, but is not very useful for very small volumes; the use of the DSC as an absolute measure of overlap is also questioned by Zou et al. [Citation18]. The consensus LADCA volume is only 5.4 cm3 which means that no overlap in a few slices results in a low DCS although the exact position of the delineated LADCA may not differ significantly seen from a clinical point of view. Hence one needs to investigate the delineated volumes visually and not solely focus on the exact value of the average DSC. However, the DSC is a useful tool for evaluating the improvements in the workshop process especially for the larger volumes as listed in .
Individualised dose planning
In most patients the radiation oncologist must balance the gain from lower risk of loco-regional recurrence with the cost of side effects from irradiating OAR. The therapy of early breast cancer patients is multimodal and complex, e.g. the systemic therapy both reduces the LRR risk and increases the risk of late morbidity after RT. It is also evident that the risk of late radiation morbidity is larger in patients operated with axillary lymph node dissection (ALND) compared to an operation based on the sentinel node technique [Citation19]. When balancing the pros and cons for RT in the individual patient we also need to consider co-morbidity as an important factor, e.g. the risk of heart morbidity is significantly higher if the patient already has had a myocardial infarction [Citation20]. In heavy smoking patients with lung diseases the field arrangement should be modified to spare as much lung tissue as possible from RT, and in some patients the RT may even cause more trouble than benefit to the patient. Thus, for example, some patients should be offered mastectomy instead of lumpectomy to avoid the adjuvant RT. In many patients treated with chemotherapy mucositis is a problem, thus the patients complain about problems with swallowing food during the last week of regional RT (unpublished observations). Since the risk of LRR in the most medial part of the medial supraclavicular region is very small it is in most patients justified to focus on sparing the eosophagus and larynx from RT [Citation21].
Conclusion
As a consequence of shifting from 2D to 3D treatment planning we have witnessed an increase in the complexity of standard adjuvant RT of early breast cancer. We are now able to target the desired volumes more accurately because they can be visualised on the planning CT scan, however, the critical OARs are also visible and it is a challenge to spare them as much as possible from radiation dose. A national delineation guideline and contouring atlas helps in achieving more precise and uniform CTVs and this enables us to create more individualised treatment plans than previously. However, it is still very important to be aware of the techniques used and volumes treated in the days of 2D, since the far majority of today's knowledge of treatment outcome is based on those techniques. A solid delineation procedure for the breast, the involved LN and OARs is also needed for future rotational therapy as all target and risk volumes really need to be delineated.
The DBCG consensus guidelines presented in this article will be used in the future trials initiated by the DBCG RT committee, and they are already by now being used in the daily routine in Denmark. Follow-up will be made prospectively on a regular basis to ensure a quality control regarding the clinical impact of the new guidelines.
Acknowledgements
BVO supported by grants from CIRRO (Center for Interventional Research in Radiation Oncology) and the Danish Cancer Society. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Overgaard M, Christensen JJ. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol 2008;47:639–53.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Post-operative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group DBCG 82b trial. N Engl J Med 1997;337:949–55.
- Ewertz M, Moe Kempel M, During M, Jensen MB, Andersson M, Christiansen P, et al. Breast conserving treatment in Denmark, 1989–1998. A nationwide population-based study of the Danish Breast Cancer Co-operative Group. Acta Oncol 2008;47:682–90.
- Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M, Ewertz M, et al. Post-mastectomy radiotherapy in Denmark: From 2D to 3D treatment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncol 2008;47:654–61.
- Martinez-Monge R, Fernandes PS, Nilendu G, Gahbauer R. Cross-sectional nodal atlas: A tool for the definition of clinical target volumes in three-dimensional radiation therapy planning. Radiology 1999;211:815–28.
- Kiricuta IC, Götz U, Schwab F, Fehn M, Neumann HH. Target volume definition and target conformal irradiation technique for breast cancer patients. Acta Oncol 2000;39: 429–36.
- Dijkema IM, Hofman P, Raaijmakers CPJ, Lagendijk JJ, Battermann JJ, Hillen B. Loco-regional conformal radiotherapy of the breast: Delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol 2004;71:287–95.
- Li XA, Tai A, Arthur DW, Buchholz TA, Macdonald S, Marks LB, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: An RTOG multi-institutional and multiobserver study. Int J Radiat Oncol Biol Phys 2009;73:944–51.
- Kirova YM, Castro Pena P, Dendale R, Servois V, Bollet MA, Fournier-Bidoz N, et al. Simplified rules for everyday delineation of lymph node areas for breast cancer radiotherapy. Br J Radiol 2010;83:683–6.
- Dice LR. Measures of the amount of ecologic association between species. Ecology 1945;26:297–302.
- Anders LC, Stieler F, Siebenlist K, Schäfer J, Lohr F, Wenz F. Performance of an atlas-based autosegmentation software for delineation of target volumes for radiotherapy of breast and anorectal cancer. Radiother Oncol 2012;102:68–73.
- International Commission on Radiation Units and Measurements. Prescribing, recording and reporting photon beam therapy (Report 50). ICRU Report. Washington D.C.: ICRU; 1993.
- Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: Method and validation. IEEE Trans Med Imaging 1994;13:716–24.
- Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer?A meta-analysis. J Clin Oncol 2000;18:1220–9.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087–106.
- Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10–8.
- Zou KH, Warfield SK, Bharatha A, Tempany CM, Kaus MR, Haker SJ, et al. Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 2004;11:178–89.
- Helms G, Kühn T, Moser L, Remmel E, Kreienberg R. Shoulder-arm morbidity in patients with sentinel node biopsy and complete axillary dissection – data from a prospective randomised trial. Eur J Surg Oncol 2009;35:696–701.
- Taylor CW, Brønnum D, Darby SC, Gagliardi G, Hall P, Jensen MB, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol 2011;100:176–83.
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer risk and prognosis. An analysis of patients from the DBCG 82 b & c randomization trials. Radiother Oncol 2006;79:147–55.
