To the Editor,
Adamantinoma (AD) is a rare indolent malignant primary bone tumor, accounting for approximately 0.4% of all primary bone tumors, affecting mainly young adults. Histologically, AD is composed of central epithelial cytokeratin positive cells and a surrounding osteo-fiberous stroma. Upon imaging studies, AD appears as an elongated, eccentric, osteolytic lesion typically located in the cortex of the mid-tibial shaft [Citation1]. AD has a propensity to metastasize to the lungs often many years after initial presentation.
Treatment of primary disease is surgical [Citation2]. In the metastatic setting only sporadic cases exist, reporting either negative [Citation3] or positive results (e.g. [Citation4,Citation5]) with systemic treatment. In the face of lacking evidence, the common treatment practice is guided by bone sarcoma protocols as this is also a primary bone disease.
We report a case of a patient with advanced AD metastatic to the lung which responded to third-line pazopanib, an oral antiangiogenic inhibitor approved in soft tissue sarcoma.
Case report
A 34-year-old woman of Middle Eastern origin presented to our institute in 2006 with complaints of pain in her left shin; workup revealed an osteo-lytic tibial lesion and biopsy showed an epithelioid neoplasm with a typical basaloid and spindle cell pattern, consistent with a diagnosis of AD. Resection of the primary tumor with allograft implantation was carried out. Follow-up was uneventful until four years later. In 2010 she began to complain of cough, chest and left shin pain. A chest CT scan performed in July 2010 demonstrated diffuse thickening of the left pleura with a large amount of pleural effusion and several solid masses in the lung parenchyma, the largest measuring 55 mm; suggestive of metastatic disease to the lungs. Thoracoscopy with pleural biopsy confirmed the diagnosis [spindle cell pattern predominant AD with relatively high mitotic activity (seven mitotic figures/10 high power fields)].
The patient was deemed inoperable and chemotherapy was initiated. She completed eight cycles of ifosfamide 7500 mg/m2, mesna 6000 mg/m2 and doxorubicin 50 mg/m2 given every 21 days with stable disease as best response. Second line chemotherapy with weekly gemcitabine 900 mg/m2 and paclitaxel 90 mg/m2 was initiated (Days 1, 8, 15 every 28 days). Stable disease was documented and treatment was withheld after four cycles as maximal response was achieved. Two months later due to progressive disease manifested by worsening dyspnea and hypercalcemia, treatment was resumed but discontinued soon after due to further disease progression.
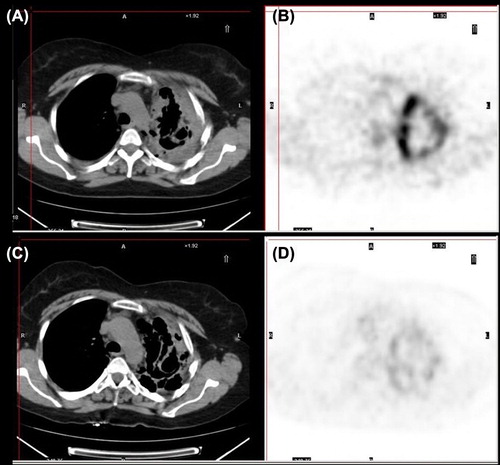
Pazopanib, a multi-tyrosine kinase inhibitor was then initiated at 800 mg per day. Prior to treatment introduction a positron emission tomography (PET)-computed tomography (CT) was performed demonstrating advanced lung metastatic disease (). Due to fatigue, a dose reduction to 600 mg per day was performed, later on to be re-increased to a full dose. After 10 weeks of treatment the patient reported symptom relief and a follow-up PET-CT (July 2012) showed a decrease in [18F]fluorodeoxyglucose avidity as well as a partial response based on RECIST criteria (). After six months of treatment the patient recently progressed clinically and radiologically.
Discussion
This article describes a case of AD of the tibia, profoundly metastatic to the lungs. Given the rarity of AD, no large scale clinical studies were performed to direct treatment; it is, however, common practice to treat this primary bone tumor similarly to bone sarcomas. Both lines of combined chemotherapy (doxorubicin, ifosfamide and gemcitabine, paclitaxel) led to stabilization of the disease.
Upon disease progression we turned to treatment with pazopanib. Pazopanib targets mainly the vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR) and KIT. Pazopanib is approved for use in renal cell carcinoma and was recently approved in metastatic soft tissue sarcoma based on a phase III trial showing an increase in progression free survival in the treatment group as compared to placebo [Citation6]. Furthermore, a case report previously demonstrated partial response of AD with lung metastases to treatment with sunitinib [Citation5]; another multi-targeted thyrosine kinase inhibitor affecting angiogenesis. Importantly, a recent study comparing sunitinib and pazopanib in renal cell carcinoma, documented fewer side effects (skin toxicity and fatigue) with pazopanib [Citation7] favoring the latter when efficacy is comparable. Interestingly, pazopanib induced a partial response, without significant side effects, in contrast to a stable disease in response to chemotherapy.
In an attempt to identify a mechanism for the response to pazopanib, we performed immuno-staining for KIT (1:200 dilution; heat-induced epitope retrieval; Cell Marque, Hot Springs AR) and PDGFR [PDGFR-alpha (1:50 dilution; no pretreatment; Santa Cruz Biotechnology, Inc; Santa Cruz, CA, USA)] on a tumor specimen from this patient that was negative. No DNA mutations insertions or deletions in the target genes KIT, PDGFRA and KDR (VEGFR2) were found as assessed by the Ion AmpliSeq™ Cancer Panel library preparation technology and sequenced by the Ion Torrent PGM platform (Life Technologies, according to manufacturer instructions). The cancer panel also included SMARCB1, a gene that when mutated is associated with hypersensitivity to pazopanib in cell cultures (see the ‘Genomics of Drug Sensitivity in Cancer’ database; (http://www.cancerrxgene.org/translation/Drug/199); this, however, was also found to be un-mutated.
We suggest that treatment with pazopanib should be considered in the setting of metastatic AD. Further molecular profiling of AD may identify the specific pathway and shed light on the mechanisms involved in sensitivity to pazopanib.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Frey SP, Hardes J, Ahrens H, Winkelmann W, Gosheger G. Total tibia replacement using an allograft (in a patient with adamantinoma). Case report and review of literature. J Cancer Res Clin Oncol 2008;134:427–31.
- Qureshi AA, Shott S, Mallin BA, Gitelis S. Current trends in the management of adamantinoma of long bones. An international study. J Bone Joint Surg Am 2000;82-A:1122–31.
- Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am 1994;76:1482–99.
- Lokich J. Metastatic adamantinoma of bone to lung. A case report of the natural history and the use of chemotherapy and radiation therapy. Am J Clin Oncol 1994;17:157–9.
- Dudek AZ, Murthaiah PK, Franklin M, Truskinovsky AM. Metastatic adamantinoma responds to treatment with receptor tyrosine kinase inhibitor. Acta Oncol 2010;49:101–4.
- Van Der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
- Motzer R, Hutson TE, Reeves J, Hawkins R, Guo J, Nathan P, et al. Randomized, open-label, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (MRCC): Results of the COMPARZ trial. 37th ESMO Congress; 2012 Sep 28 – Oct 2; Vienna, Austria.