Abstract
Background. Breast cancer survivors may experience adverse effects of cancer and/or treatment years after completion of therapy, which can considerably decrease quality of life (QoL). Little is known about the time course of QoL in breast cancer survivors beyond the fifth year post-diagnosis, when routine follow-up care has usually terminated. We therefore explored in detail whether and to what extent restrictions in breast cancer survivors persist and whether changes or aggravations in QoL occurred over time. Material and methods. QoL was assessed 1, 3, 5, and 10 years post-diagnosis in a population-based cohort of initially 387 female breast cancer patients from Saarland (Germany), using the EORTC QLQ-C30 and QLQ-BR23. Time course of QoL over 10 years post-diagnosis was assessed for survivors and survivors’ QoL was compared cross-sectionally to the German general population after adjustment for age. Results. A total of 182 out of 238 patients alive (76.5%) responded in the 10-year, 160 patients (67.2%) participated in all follow-ups. Although breast cancer survivors and controls reported comparable general health and overall QoL, survivors reported significantly more restrictions on most functioning and symptom scales at each follow-up. Detriments in various QoL dimensions (e.g. physical and social functioning; pain, financial difficulties) aggravated from year 5 to 10. Generally, restrictions were largest for the youngest survivors. Conclusion. Relevant restrictions in QoL persist over years in breast cancer survivors and affect predominantly younger women. The aggravation of restrictions in QoL beyond the fifth year may indicate deficits in health care and psychosocial support of breast cancer patients after completion of routine follow-up care.
Breast cancer is the most common cancer among women, with an annual incidence of approximately 1.4 million worldwide [Citation1]. Due to advances in early detection, treatment, and follow-up care, relative five-year survival rates for breast cancer are currently estimated at 79% in Europe and 90% in the US [Citation2], resulting in a constantly growing number of long-term survivors (≥ 5 years post-diagnosis) [Citation3]. Since more and more women survive breast cancer, quality of life (QoL) has become a major focus in research and clinical practice [Citation4].
Previous studies indicate that breast cancer survivors continue to experience adverse effects of cancer and/or treatment years after completion of therapy [Citation5], including pain [Citation5,Citation6], fatigue [Citation5,Citation7], depressive symptoms [Citation6], fear of recurrence [Citation8], and poor sexual functioning [Citation5,Citation9].
However, most results on QoL in breast cancer survivors are based on cross-sectional studies, exhibiting a potential risk of selection bias/period effects when comparing short- and long-term survivors, and do not allow an examination of the course of QoL over time. In addition, only few longitudinal studies following up patients beyond the fifth year after diagnosis have been carried out [Citation10–12], when routine follow-up care has been completed for most breast cancer patients.
Given the increasing number of long-term survivors, the potential late effects of the disease and/or treatment, and the limited knowledge on the time course of QoL in breast cancer survivors, we explored in detail whether and to what extent restrictions in breast cancer survivors persist in the long run and whether changes or aggravations in QoL occur over time.
Material and methods
Study design/study population
Data in the present study was retrieved from a larger longitudinal, population-based cohort study in Saarland (state in southwest Germany; population approx. 1 million), studying patterns of diagnostic processes, QoL, and survival in breast cancer as well as in colorectal, and gastric cancer patients. The study was initiated in 1996, with data collections at baseline (a few days after diagnosis) and four follow-up time-points after one (FU1), three (FU3), five to seven (FU5), and 10 to 12 (FU10) years post-diagnosis. Details on study population and data collection have been described elsewhere [Citation13].
In brief, all hospitals offering in-patient treatment in the study region participated in patient recruitment. Eligibility criteria at baseline included residence in Saarland, histologically confirmed invasive breast, colorectal, or gastric cancer, age 18–80 years, and sufficient knowledge of the German language. Due to the high work load of physicians, not all eligible patients could be approached and referred to the study center. Overall, 387 women with breast cancer participated in the baseline interview and constitute the study population for the present analysis. The participants represented approximately 50% of all incident breast cancer cases during recruitment and did not substantially differ from the source population on basic socio-demographic characteristics with the exception of a slightly higher proportion of younger patients. For the four follow-up assessments, questionnaires were sent to participants alive who had given and not withdrawn permit to be re-contacted. Non-responders were mailed up to two reminders, followed by a reminder phone-call.
The study was approved by the ethics committees of the University of Heidelberg and the Medical Association of Saarland. Written informed consent was obtained from all participants.
Data collection and measurements
At baseline, standardized in-person interviews were conducted to assess socio-demographics, comorbidity, and details of the initial diagnostic work-up. Medical data, including information on tumor stage at diagnosis and initial therapy, was obtained from hospital records and discharge letters.
QoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30 items (EORTC QLQ-C30) [Citation14] and the breast cancer specific module QLQ-BR23 [Citation15] in all follow-up rounds. Both measures are brief, validated self-reporting cancer-specific measures of health-related QoL, consisting of functional as well as symptom scales. On both instruments, higher scores on the functioning scales represent better functioning, whereas higher symptom scores indicate more severe symptoms.
Information regarding tumor recurrence and vital status (non-responders) was obtained from the Saarland Cancer Registry.
Individual level QoL-data of the German general population, collected from 968 women in 1998 served as an external reference. Participants were randomly selected by random-route-technique and were interviewed in their homes by trained interviewers. The interview included, among others, questions on socio-demographics and the EORTC QLQ-C30 [Citation16].
Statistical methods
Scoring of the EORTC QLQ-C30 and QLQ-BR23 was performed according to the EORTC scoring manual. All scores were linearly transformed to a 0 to 100 scale. Differences in mean QoL scores larger than 10 points were considered clinically relevant [Citation17]. All analyses were performed using the SAS statistical software (Version 9.2).
Cross-sectional analyses
Analysis of variance (ANOVA) was used to compare QoL of patients who responded in FU10 (N = 182) to QoL of references, stratified by age-group (18–49, 50–64, 65+). To adjust the comparisons of survivors and references for age, weighted means and standard errors were computed for references according to the age distribution (five year age-groups) of survivors. A sample size of 182 was sufficient to detect a difference of 10 points between survivors and references at p < 0.05 with a power of over 90%.
Longitudinal analyses
The time course of QoL from FU1 to FU10 was examined for full-responders (patients participating in all follow-ups) (N = 160). Only full-responders were included to minimize bias due to drop-outs. Participants dropping out (specifically deceased participants) might lead to an observed improvement of QoL over time, which could be misleading. Repeated measures ANOVA with the factor follow-up round and post-hoc comparison between follow-ups were computed to assess changes in QoL scales over time. In addition, QoL scores of survivors and references were compared cross-sectionally (ANOVA, age- adjusted) at each follow-up to provide a more complete picture of survivors’ QoL at each time-point.
Results
Participant characteristics
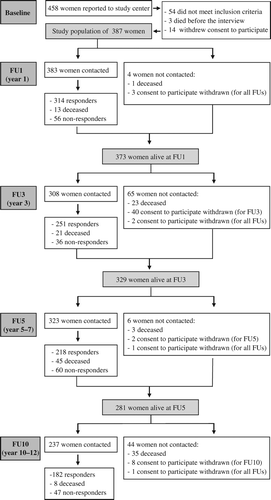
Of the 387 participating women diagnosed with breast cancer in 1996–1998, 238 were still alive at FU10, of whom 182 participated in FU10 (76.5% of survivors alive at FU10) and 160 women participated in all follow-ups (67.2% of survivors alive at FU10). Response rates in the three earlier QoL assessments were 84.2% (314 of 373 women alive at FU1), 76.3% (251 of 329 women alive at FU3), and 77.6% (218 of 281 women alive at FU5) for FU1, FU3, and FU5, respectively (). The mean age of participants at baseline was 55.6 (FU10 responders) and 55.4 years (full responders). All but one of the participants of FU10 were diagnosed with local or regional breast cancer at baseline, but almost 20% experienced a recurrence during the follow-up period. To determine the potential impact of non-random missing data mechanisms, patient and disease characteristics of responders, non-responders, and deceased patients were compared. At FU10, there were no significant differences between responders and non-responders, except for responders being more likely to have received chemotherapy (46% vs. 31%, p = 0.05). Global health/QoL was comparable at the first QoL-assessment (FU1) for all groups, with the exception of subjects deceased between FU1 and FU10 reporting worse QoL than patients alive at FU10 [mean± SD: 60.3 ± 23.7 (deceased) vs. 62.6 ± 18.4 (non-responders) and 68.7 ± 21.1 (responders), p < 0.0001] (). Full responders differed from partial responders (participation in less than all follow-ups) alive at FU10 only on chemotherapy, with full responders being more likely to have undergone it (47% vs. 33%, p = 0.03) [data for partial responders (n = 80) not shown].
Table I. Characteristics of study population by response statusa.
Cross-sectional analyses
QLQ-C30.
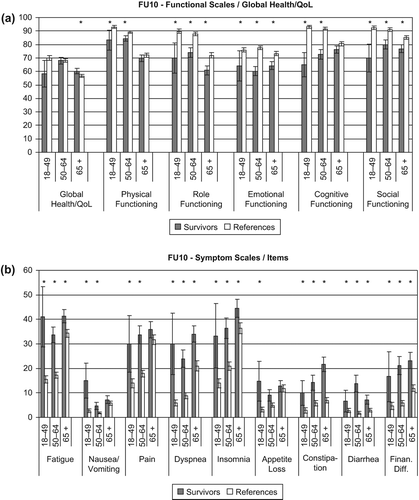
Overall, breast cancer survivors 10 years past diagnosis and references from the general population reported comparable general health and overall QoL (). Only for women older than 65 years, differences reached statistical significance, with survivors reporting slightly, although not clinically relevant, better global health/QoL than references. In contrast, younger survivors tended to report lower global health/Qol than references albeit the difference did not reach statistical significance. Although breast cancer survivors’ and references’ overall health/QoL was comparable, survivors experienced significantly more functional restrictions and more symptoms on all other scales and single items of the QLQ-C30 (). Differences between survivors and references were statistically significant (p < 0.05) for all age-groups on all functioning scales, except for physical and cognitive functioning, where differences were not significant for the oldest age-group. Also, the majority of differences on the symptom scales and single items were statistically significant. Exceptions were the middle and the oldest age-group, which did not differ significantly from references on appetite loss; also differences were not statistically significant for the oldest age-group on nausea/vomiting and pain. The majority of differences in functioning and symptom severity were clinically relevant. Functional impairments were the gravest for role, cognitive, and social functioning, with differences in mean scores between survivors and references aged below 50 years of 19.9, 28.2, and 12.2, respectively (p < 0.0001). Among the symptoms, the greatest differences between survivors and references were found for fatigue and dyspnea, with mean differences between survivors and references aged below 50 years of 25.7 (p < 0.0001) and 24.1, respectively (p = 0.003). Generally, restrictions were largest for the youngest age-group. Scores and p-values can be found in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.774461).
Longitudinal analyses
QLQ-C30.
Global health/QoL was comparable for survivors and references at each follow-up time-point over 10 years, with breast cancer survivors reporting slightly better QoL (). Differences were neither statistically significant nor clinically relevant. For survivors, QoL showed a steady decline from a mean score of 84.8 at FU1 to a mean score of 75.6 at FU10 (overall change p = 0.02), with differences to references’ QoL slightly increasing over the 10-year time period. In contrast to overall QoL, breast cancer survivors reported worse functioning () and more symptoms () than references at each follow-up. Differences between survivors and references were statistically significant for all functioning and symptom scales and single items at all follow-ups, except for physical functioning and appetite loss, which were only significantly different at FU10 and nausea/vomiting and pain, with differences below statistical significance at FU1 and FU3, respectively. For emotional functioning, fatigue, and insomnia, differences were clinically relevant at all time-points; differences on most remaining scales and single items were clinically relevant at a minimum of one follow-up. Differences between survivors and references even increased from five to 10 years post-diagnosis for physical, emotional, cognitive, and social functioning as well as nausea/vomiting, pain, dyspnea, appetite loss, constipation, and financial difficulties.
Figure 3. Longitudinal development (means and standard errors) of QLQ-C30 functional scales and global health/QoL for survivors that responded in each follow-up (solid line) and cross-sectional comparisons with references adjusted for age (dots). P-values pertain to differences between survivors and references at each follow-up.
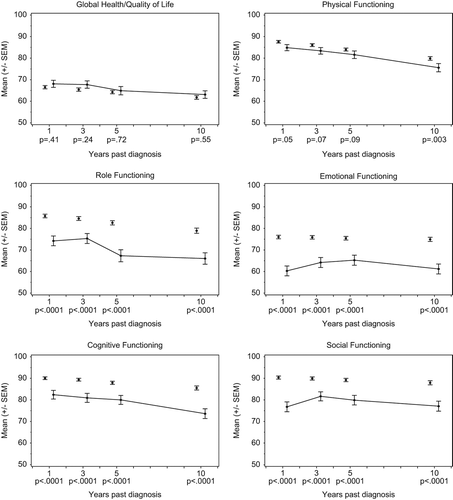
Figure 4. Longitudinal development (means and standard errors) of QLQ-C30 symptom scales and single items for survivors that responded in each follow-up (solid line) and cross-sectional comparisons with references adjusted for age (dots). P-values pertain to differences between survivors and references at each follow-up.
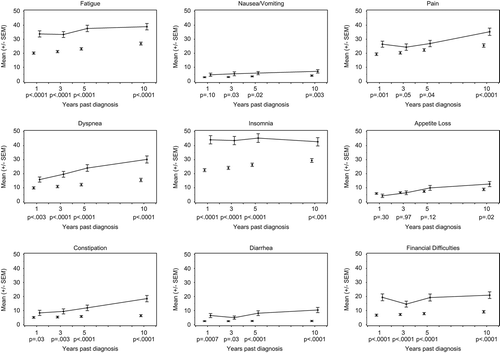
In general, functioning tended to deteriorate and reported symptoms increased for survivors from FU1 to FU10. For emotional and social functioning, breast cancer survivors reported an increase in functioning from FU1 to FU3/FU5 of 3.9 and 4.8, respectively, followed by a decrease until FU10 to a functioning score comparable to that at FU1 (emotional functioning: 61.2, social functioning: 77.1). Overall changes from FU1 to FU10 in survivors` physical, role and cognitive functioning, as well as in global health/QoL, fatigue, pain, dyspnea, appetite loss, pain, constipation, diarrhea, and financial difficulties were statistically significant. Changes from FU1 to FU10 in mean scores were clinically relevant for dyspnea (FU1: 15.8, FU3: 19.6, FU5: 24.0, FU10: 30.1, overall change p < 0.0001) and constipation (FU1: 8.3, FU3: 9.3, FU5: 11.9, FU10: 18.4, overall change p < 0.0001). Scores and p-values can be found in Supplementary Table II (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.774461).
QLQ-BR23.
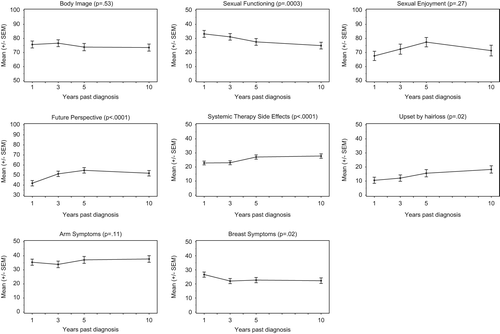
Sexual functioning decreased over time, whereas arm symptoms, systemic therapy side effects, and being upset by hair loss slightly increased. A decline in breast symptoms (FU1: 26.6, FU3: 22.0, FU5: 22.7, FU10: 22.3, overall change p = 0.02), as well as an increase in future perspective (FU1: 41.8, FU3: 51.2, FU5: 54.7, FU10: 51.8, overall change p < 0.0001) was reported, whereas body image remained relatively stable (FU1: 75.7, FU3: 76.6, FU5: 73.8, FU10: 73.5, overall change p = 0.53) from FU1 to FU10. Even though sexual functioning decreased over time, sexual enjoyment increased from FU1 to FU5 and at FU10 dropped to a degree comparable to that reported at FU1. Overall changes over time were statistically significant, with the exception of body image, sexual enjoyment, and arm symptoms ().
Additional/subgroup analysis
The overall pattern of longitudinal changes in QoL in breast cancer survivors did not change when we reran the analysis within subgroups according to age, stage, tumor recurrence, therapy, and education (data not shown). Also, the pattern remained the same when data of all survivors, including partial responders, were used for the comparison instead of data of full-responders only (Supplementary Table III, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.774461).
Discussion
As illustrated, knowledge regarding long-term QoL in women with breast cancer is very limited. Results of our study indicate that, compared to the general population, breast cancer survivors not only experience clinically relevant restrictions in several QoL dimensions 10 years after diagnosis, but some restrictions even increase in the long run. Restrictions were greatest for role, cognitive and social functioning, and fatigue, which is in accordance with results obtained within other studies on long-term breast cancer survivors [Citation5,Citation11]. Generally, the youngest age-group seemed to experience the highest level of restrictions, representing a continuation of the pattern found in our previous results on the same cohort [Citation18,Citation19]. Global health/QoL was comparable to QoL of the general population and did not significantly change over time, supporting previous results [Citation20,Citation21].
Changes over time were observed for physical functioning, with survivors reporting a significant decrease over time, which is in accordance with prior results [Citation11,Citation22]. In contrast, previously reported findings of an increase in functioning in the psychological domain [Citation12,Citation23,Citation24] and role functioning [Citation24] are not completely supported by our study. A steady increase in functioning was only observed for future perspective, suggesting a growing optimistic outlook from one to 10 years post-diagnosis. A reason for these conflicting results might be the length of follow-up, with previous studies mostly following up women for less than five years. Our findings do indicate a slight increase in emotional as well as social functioning from one to five years post- diagnosis, which is followed by a decrease. This decrease could partly be due to the normal aging process, which might not be covered to the same extent when examining patients over shorter follow-up periods. The results might also indicate specific deficits in healthcare and/or psychosocial support services for breast cancer survivors after five years post-diagnosis, when routine follow-up has been completed for most survivors. Bloom et al. found physical and social functioning to decrease from five to 10 years post-diagnosis and psychological functioning to be relatively stable [Citation10], which is in line with our findings. Moreover, Sagen et al. found a significant decrease in functioning from prior to surgery to five years post-diagnosis, which further supports our findings [Citation22].
In addition to the contrasting findings on functioning, the general increase in symptoms shown by our results is not completely supported by earlier studies. Even though some symptoms, like fatigue, were found to increase slightly [Citation24], most previous studies observed a decrease in symptoms [Citation10,Citation22]. Again, these contradictory results might be due to the shorter follow-up periods of previous studies, disguising age-related changes. Also, as different symptoms were measured by different studies, results cannot be compared directly.
Cross-sectional differences between survivors and references increased from FU1 to FU10 on most scales and single items. Also, at each follow-up, survivors scored worse on functioning and symptom severity than references. These results strongly suggest that changes in QoL dimensions are not only due to the normal aging process, but can also be attributed to breast cancer, possibly leading to different care needs of this population. As comparisons were cross-sectional, causality could not be determined. Treatment-related symptoms, like arm swelling, systemic therapy side effects, and being upset by hair loss, slightly increased or persisted from one to 10 years post-diagnosis. Similar results have been found in other studies, reporting arm symptoms [Citation5,Citation22] or hair loss [Citation25] among long-term breast cancer survivors. Further in depth study of these symptoms, specifically those typical for the treatment phase, is needed to determine whether answers generally pertain to current symptoms or questions are answered in memory of past experiences during treatment.
Despite clinically relevant restrictions in several QoL dimensions, global QoL scores do not seem to reflect the longitudinal changes of symptoms and functioning, which might be explained by adaptive processes (response shift) influencing global QoL scores, but not functioning and symptoms. Response shift has been defined as a change in the meaning of one's self-evaluation of a construct of interest (e.g. QoL) as a result of changes in internal measurement standards or values, or a redefinition of the target construct [Citation26]. As functioning and symptom scores are probably not highly influenced by response shift, changes in these areas can be considered real.
One might argue that restricting our analysis only to those women who participated in all follow-ups might introduce attrition bias. However, our findings did not change when we integrated the results of earlier and partial responders. As more responders than non-responders received chemotherapy, symptoms typically associated with chemotherapy (e.g. restrictions in cognitive functioning) might have been overestimated. Also, late effects may differ depending on therapy received. For example, different chemotherapy regimes [Citation27] or different types of surgery [Citation13,Citation28] might lead to different long-term side effects. In addition, therapy has constantly evolved over the past decade. New chemotherapy and endocrine therapy regimes have been introduced and a shift occurred from mastectomy to breast conserving therapy and from axillary lymph node dissection (ALND) to sentinel node biopsy (SNB) [Citation29]. Late-effects typically associated with the up-to-date state of the art therapy might therefore be underrepresented (e.g. peripheral neuropathy due to taxanes [Citation30]) and effects associated with the formerly more common therapies overrepresented (e.g. arm symptoms due to ALND [Citation31]). Thus, not all of our results might be applicable to breast cancer survivors in general or those diagnosed more recently. Nevertheless, as some symptoms and detriments seem not to be influenced mainly by therapy regimes, results of our study are not only relevant for women diagnosed in the inclusion period of our study, but also for women diagnosed at later time-points. Another possible limitation of our study is the use of historic references [Citation16], as health-related QoL in the German population might have changed since 1998. However, the study group providing the population data has performed a similar data collection in 2012 and confirmed that population norms have not changed considerably (personal communication, data unpublished). Also, as references’ QoL was measured at only one time-point, period effects might come into play when comparing QoL of survivors and references, as survivors are compared to references from different birth cohorts at different follow-ups.
When comparing QoL of survivors and the general population, we assumed that QoL of residents of Saarland is comparable to QoL of the included representative sample of the German population. There are no data available to determine comparability of QoL of residents of Saarland and the German general population, but variables associated with QoL like income per resident and the number of one-person households are comparable, with the exception of residents of Saarland being slightly lower educated. Moreover, as analyses comparing QoL of breast cancer survivors from another German state to the same reference population yielded similar results [Citation32], QoL of the population of individual states is likely to be comparable to QoL of the German general population as a whole. A further possible caveat of the study is the lack of a baseline assessment of QoL and the baseline recruitment rate of about 50%, which might lead to under- or overestimation of QoL. Statistical significance was set at p < 0.05. As most of our results are significant on the p < 0.01 level, no further adjustment for multiple testing was employed.
Despite these limitations, our study has the strength of a prospective, longitudinal assessment of QoL, allowing the examination of QoL-development over time, thus minimizing possible bias due to period effects when comparing short- and long-term survivors. Also, there are only few longitudinal studies, with the study carrying out the maximum follow-up being limited to young breast cancer survivors [Citation10]. Keeping in mind the current demographic aging, our results are an important addition to existing knowledge, as older breast cancer patients are included and results indicate persistence of restrictions in this group of survivors. In spite of the limitations concerning the reference data, another strength of our study is the comparison of QoL of survivors and the general population, permitting a differentiation between restrictions associated with the normal aging process and those that might have additionally been influenced by cancer and/or its treatment.
In conclusion, breast cancer survivors continue to experience restrictions in a variety of QoL dimensions even years after diagnosis, specific symptoms persist or even increase over time, and restrictions in QoL appear to not only being associated with the normal aging process but also with cancer and/or its treatment. Therefore, researchers and clinicians should be aware of restrictions experienced by long-term breast cancer survivors to further optimize care for this growing population.
Supplementary Tables I–III
Download PDF (42.6 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2008.
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: A 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol 2007;8:784–96.
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010.
- Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol 2007;46:417–32.
- Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5 +years) adult cancer survivors. Psychooncology 2007;16:691–706.
- Paskett ED, Alfano CM, Davidson MA, Andersen BL, Naughton MJ, Sherman A, et al. Breast cancer survivors’ health-related quality of life: Racial differences and comparisons with noncancer controls. Cancer 2008;113: 3222–30.
- Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors?A systematic review of the literature. Breast Cancer Res Treat 2008;112:5–13.
- Lee-Jones C, Humphris G, Dixon R, Hatcher MB. Fear of cancer recurrence-a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology 1997;6:95–105.
- Biglia N, Moggio G, Peano E, Sgandurra P, Ponzone R, Nappi RE, et al. Effects of surgical and adjuvant therapies for breast cancer on sexuality, cognitive functions, and body weight. J Sex Med 2010;7:1891–900.
- Bloom JR, Stewart SL, Oakley-Girvan I, Banks PJ, Shema S. Quality of life of younger breast cancer survivors: Persistence of problems and sense of well-being. Psychooncology 2012;21:655–65.
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst 2002;94:39–49.
- Dorval M, Maunsell E, Deschenes L, Brisson J. Type of mastectomy and quality of life for long term breast carcinoma survivors. Cancer 1998;83:2130–8.
- Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: A population-based study. J Cancer Res Clin Oncol 2008;134:1311–8.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol 1996;14:2756–68.
- Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer 2001;37:1345–51.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Persistence of restrictions in quality of life from the first to the third year after diagnosis in women with breast cancer. J Clin Oncol 2005;23:4945–53.
- Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer 2004;40:673–80.
- Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer 2009;115:4001–9.
- Peuckmann V, Ekholm O, Rasmussen NK, Moller S, Groenvold M, Christiansen P, et al. Health-related quality of life in long-term breast cancer survivors: Nationwide survey in Denmark. Breast Cancer Res Treat 2007;104:39–46.
- Sagen A, Karesen R, Sandvik L, Risberg MA. Changes in arm morbidities and health-related quality of life after breast cancer surgery – a five-year follow-up study. Acta Oncol 2009;48:1111–8.
- Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: Quality of life of young breast cancer survivors. Psychooncology 2004;13:147–60.
- Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J 2004;10:223–31.
- Deimling GT, Sterns S, Bowman KF, Kahana B. The health of older-adult, long-term cancer survivors. Cancer Nurs 2005;28:415–24.
- Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med 1999;48:1507–15.
- Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: A database study. Br J Cancer 2011;105(Suppl 1):S29–37.
- Ohsumi S, Shimozuma K, Morita S, Hara F, Takabatake D, Takashima S, et al. Factors associated with health-related quality-of-life in breast cancer survivors: Influence of the type of surgery. Jpn J Clin Oncol 2009;39 :491–6.
- Van Ewijk RJ, Schwentner L, Wockel A, Konig J, Kreienberg R, Blettner M. Trends in patient characteristics, treatment and survival in breast cancer in a non-selected retrospective clinical cohort study of 2,600 patients. Arch Gynecol Obstet 2013;287:103–10.
- Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Support Care Cancer 2012; 20:3355–64.
- Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment – a nationwide study of prevalence and associated factors. Breast 2010; 19:506–15.
- Waldmann A, Pritzkuleit R, Raspe H, Katalinic A. The OVIS study: Health related quality of life measured by the EORTC QLQ-C30 and -BR23 in German female patients with breast cancer from Schleswig-Holstein. Qual Life Res 2007;16:767–76.