Abstract
Purpose. The angiogenesis inhibitor pazopanib has been approved for the treatment of advanced renal cell cancer (RCC) and soft tissue sarcoma. Severe and fatal hepatotoxicity has been observed in its clinical studies. This analysis was conducted to determine the risk of liver toxicity by a systematic review and meta-analysis of clinical trials. Patients and methods. Databases from PubMed, Web of Science and abstracts presented at ASCO meetings up to January, 2012 were searched to identify relevant studies. Eligible studies included prospective trials of cancer patients treated with pazopanib starting at 800 mg daily. Summary incidence rates, relative risks, and 95% confidence intervals (CIs) were calculated using a fixed- or random-effects model. Results. A total of 1478 patients from 10 clinical trials were included. The incidences of all-grade aspartate aminotransferase (AST), alanine transaminase (ALT), and bilirubin elevation were 39.6% (95% CI 31.2–48.6%), 41.4% (95% CI 34.1–49.0%), and 24.8% (95% CI 16.3–35.3%), respectively. The incidences of high-grade (Grade 3 and 4) AST, ALT and bilirubin elevation were 6.9% (95% CI 5.5–8.6%), 9.4% (95% CI 7.8–11.4%), and 3.4% (2.4–5.0%), respectively. In comparison with placebo, pazopanib significantly increased the risk of high-grade AST elevation (RR 6.56, 95% CI 2.04–21.07, p = 0.002) and ALT elevation (RR 4.33, 95% CI 1.88–10.0, p = 0.001). However, the risks of high-grade bilirubin elevation (RR 1.31, 95% CI 0.47–3.64) and fatal hepatotoxicity (RR 2.51, 95% CI 0.12–51.91, p = 0.55) were not significantly elevated. Conclusion. The use of pazopanib was associated with a significantly increased risk of severe non-fatal hepatotoxicity in cancer patients.
Vascular endothelial growth factor (VEGF) plays an important role in tumor growth, invasion, and metastasis by promoting angiogenesis [Citation1–3]. Inhibition of the VEGF signaling pathway has become a major approach to cancer therapy. Pazopanib (Votrient™, GlaxoSmithKline, Philadelphia, PA, USA) is a second-generation multi-targeted tyrosine kinase inhibitor (TKI) with highly selective activity against VEGF receptor-1, -2, -3, platelet-derived growth factor receptors -α and -β, and stem cell factor receptor c-kit [Citation4]. Its efficacy has been demonstrated in a placebo-controlled phase III study for patients with treatment-naïve or cytokine- pretreated advanced renal cell carcinoma (RCC) and has received US Food and Drug Administration (FDA) approval in October 2009 for that indication [Citation5–7]. Pazopanib has also shown clinical activity against several other tumor types including soft tissue sarcomas, cervical cancer, and breast cancer [Citation6–9]. Recently, it has been approved for the treatment of soft tissue sarcoma [Citation10]. Numerous phase II and III clinical trials have evaluated its growing clinical potential [Citation5,Citation7–9,Citation11–18].
Compared to the most studied and widely used first generation VEGF receptor TKIs sorafenib and sunitinib, clinical trials with pazopanib have demonstrated some distinct differences in toxicity profiles. For the RCC trials with pazopanib, the most frequently observed adverse events (AEs) or laboratory abnormalities were diarrhea, changes in hair color, hypertension, nausea, fatigue, alanine transaminase (ALT) and aspartate aminotransferase (AST) elevation. Two of the most common high-grade (Grade 3 or higher) AEs included the elevation of transaminases ALT and AST [Citation5,Citation18]. Transaminase elevations were usually early in the course of treatment, reversible, and patients were generally asymptomatic. However, severe liver damage can occur, leading to treatment interruptions, significant morbidity, and even fatal adverse events (FAEs). In the phase III trial consisting of 435 patients (290 pazopanib; 145 placebo) with advanced RCC, four FAEs were found in the intervention arm [Citation5]. Of these, two were attributed to abnormal hepatic function related to study treatment. Additionally, of the 1830 pazopanib treated patients in the manufacturer's safety database, two FAEs may have been related to pazopanib-induced liver failure [Citation19].
The role of pazopanib in the development of liver toxicity has yet to be defined. Current understanding of its risk based on individual trial is limited due to small sample size and patient selection in these clinical studies. Therefore, we conducted a systematic review of published phase II and III clinical trials, and combined relevant studies for a meta-analysis to evaluate the overall risk of liver toxicity with pazopanib.
Patients and methods
Data source
A comprehensive, independent review of citations from PubMed between 1 January 1966 and 30 January 2012 was conducted using the key words “pazopanib”, “votrient”, “cancer”, and “trial.” The search was limited to clinical trials involving human subjects. Additionally, we searched all abstracts and virtual meeting presentations using the terms “pazopanib” and “cancer” from the American Society of Clinical Oncology conferences (http://www.asco.org) between January 2000 and January, 2012. Independent searches using EMBASE, Web of Science database, between 1 January 1966 and 30 June 2012 were also conducted to ensure trials were not missed in the analysis. Each publication and/or abstract was examined in detail to ensure the most recent data from the respective clinical trial was incorporated in our results, particularly when duplicate publications were discovered. The safety data from pazopanib's updated manufacturer's package insert was also reviewed to identify pertinent information. Study investigators and the manufacturers of pazopanib were contacted when data was not clear.
Study selection
The primary goal of this study was to determine the risk of liver toxicity in cancer patients treated with pazopanib. Pazopanib has been approved for use in patients with advanced RCC as a single agent at the starting dose of 800 mg by mouth daily. Thus, clinical trials using pazopanib at the standard dose in cancer patients with available data on liver toxicity were selected for the analysis. Phase I trials were excluded due to variation in dosage. Studies that met the following criteria were included: 1) prospective clinical trials involving cancer patients; 2) assignment of participants to pazopanib treatment at a starting dose of 800 mg daily; and 3) available data regarding events, sample size, or incidence of hepatotoxicity. The quality of studies was assessed using criteria including completeness of follow-up and objectivity of outcome measurements.
Data extraction and clinical endpoints
Details extracted from the studies included study characteristics, number of patients, patient characteristics, treatment information, and type of malignancy from the selected trial. Incidences, or events of all-grade (Grade 1–5) and high-grade (Grade 3–5) AST and ALT elevation, bilirubin elevation, liver-related FAEs, as well as sample sizes were extracted from the safety profile of each trial. Independent data extraction was executed by three reviewers and discrepancies were resolved by discussion and consensus. Hepatotoxic events in these studies were assessed and recorded according to version 3 of the National Cancer Institute’s Common Terminology Criteria (NCI-CTC) for Adverse Events, which have been widely used in cancer clinical trials. Grades 1 and 2 AST and ALT elevations are defined by an increase of ≤ 2.5 × ULN (upper normal limit) and > 2.5–5.0 × ULN, respectively. High-grade liver toxicity, specifically Grades 3 and 4 transaminase elevation, was defined as ≥ 5.0–20.0 × ULN and > 20.0 × ULN, respectively. The grading for hyperbilirubinemia was defined as the following: Grade 1, > ULN-1.5 × ULN; Grade 2, > 1.5–3.0 × ULN; Grade 3, > 3.0–10.0 × ULN; Grade 4, > 10.0 × ULN. Grade 5 toxicity is not defined for AST, ALT, and bilirubin elevation.
Statistical analysis
All statistical analyses were performed using Comprehensive Meta-analysis software, version 2 (Biostat, Englewood, NJ, USA), as described in our previous studies [Citation20,Citation21]. Briefly, for the calculation of incidence, the number of patients with AST, ALT or bilirubin elevation (both high-grade and all-grade) and the number of patients receiving pazopanib were extracted from the selected clinical trials; the proportion of patients with AST and ALT elevation and 95% exact confidence intervals (CI) were derived from each study. To calculate relative risk (RR), patients assigned to pazopanib were compared only to those assigned to control treatment in the same trial.
For the meta-analysis, we used fixed-effects (weighted with inverse variance) or random-effects models to determine summary results. For each meta-analysis, the Cochran's Q statistic and I2 score were first calculated to assess the heterogeneity among the proportions of the included trials. For the p-value of Cochran's Q statistic < 0.1, the assumption of homogeneity was deemed invalid, and a random-effects model was reported. The causes of heterogeneity were also explored in this context. Otherwise, results from the fixed-effects model were reported. A two-tailed p-value < 0.05 was judged as statistically significant. We used the Begg's and Egger's tests to determine the presence of publication bias regarding primary endpoint (all-grade AST and ALT elevation). A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Search results
Our literature search yielded 375 potentially relevant clinical trials on pazopanib. After excluding review articles, duplicate studies, phase I studies, case reports, case series, pharmacokinetic studies, editorials, meta-analysis, and observational studies, a total of 10 clinical trials were selected for the purpose of analysis (). These trials include eight phase II and two phase III studies, and their characteristics including number for analysis, underlying malignancies, trial phase, pazopanib dosing, and NCI-CTC versions are listed in .
Table I. Characteristics of trials included in the meta-analysis.
Study quality
Randomized treatment allocation sequences were generated in all RCT trials. Seven trials were single-arm, open-label phase II studies [Citation7,Citation9,Citation11–13,Citation16,Citation18]. Two trials were placebo-controlled, randomized, double-blind, global, multicenter, phase III studies [Citation5,Citation17]. The remaining trial was a randomized, open-label, multicenter, phase II trial consisting of a combination treatment of pazopanib plus placebo versus daily oral pazopanib or daily oral placebo as monotherapy [Citation8]. This study initially randomly assigned patients to the combination or monotherapy arms. Later, the protocol was amended after results of a formal interim analysis indicated that the futility boundary for efficacy of the combination therapy compared with placebo monotherapy arm was crossed and that there was an imbalance in toxicity between the combination arm and the monotherapy arms. On the basis of these observations, combination therapy was discontinued. Transaminase or bilirubin elevation was assessed and recorded according to National Cancer Institute's common toxicity criteria version 3 as described above. The quality of all the trials was acceptable.
Patients
A total of 1478 patients (pazopanib, n = 1134; control, n = 344) with a variety of solid tumors from 10 clinical trials were included for the analysis. All patients were ≥ 18 years of age with histologically or cytologically confirmed malignancies. Most patients had a baseline Eastern Cooperative Oncology Group status between 0 and 1 with measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST). Patients were required to have well- controlled blood pressures with adequate hepatic, renal, and hematologic functions. Patients were excluded for serious co-morbidities including CNS metastasis, QTc prolongation (defined as a QTc interval of ≥ 470 ms), class III/IV heart failure, history of cerebrovascular accident, myocardial infarction, unstable angina, cardiac angioplasty/stenting, or untreated venous thrombosis within six months of screening. Underlying malignancies included RCC (two studies) [Citation5,Citation18], soft tissue sarcomas (two studies) [Citation7,Citation17], recurrent cervical cancer (one study) [Citation8], metastatic breast cancer (one study) [Citation9], recurrent glioblastoma (one study) [Citation11], ovarian cancer (one study) [Citation12], metastatic NSCLC (one study) [Citation13], and thyroid cancer (one study) [Citation16]. Seven trials had the design of open-label single arm in which patients received pazopanib 800mg once daily [Citation7,Citation9,Citation11–13,Citation16,Citation18]. In two trials, patients were randomly assigned to either a control or pazopanib group [Citation5,Citation17]. In one trial, patients were assigned to either control, pazopanib, or pazopanib plus control combination arm [Citation8].
Incidence of AST elevation
Eight clinical trials included data pertaining to all-grade AST elevation and were analyzed. A total of 860 patients with solid tumor types were available for analysis. Incidences ranged from 16.7% to 53.8% with the highest incidence observed in a phase II trial in patients with RCC [Citation18], and the lowest in a phase II trial in patients with recurrent ovarian cancer [Citation12]. Among the patients who were administered pazopanib, the summary incidence of all-grade AST elevation was 39.6% (95% CI 31.2–48.6%), using a random-effects model (Q = 37.785, I2 = 81.474, p < 0.001).
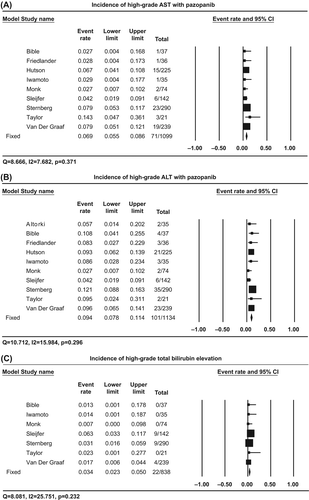
Nine trials reported incidences of high-grade (Grade III and/or IV) AST elevation, with the highest incidence (14.3%) observed in a phase II metastatic breast cancer trial [Citation9], and the lowest incidence (2.7%) seen in two distinct trials; a phase II recurrent cervical cancer trial [Citation8], and a phase II advanced differentiated thyroid cancer trial [Citation16]. Using a fixed-effects model (Q = 8.666, I2 = 7.682, p = 0.371), the summary incidence of high-grade AST elevation was 6.9% (95% CI 5.5–8.6%, ).
Figure 2. Incidences of high-grade AST, ALT, and bilirubin elevation associated with pazopanib. Summary incidences were calculated using a fixed-effects model for high-grade AST (A), ALT (B), and bilirubin elevation (C). Incidence for each study is displayed numerically on the left and graphically on the right. Total events and sample sizes are also displayed for each study. For study name, the first author's name was used to represent each trial. For each trial: filled-in square, incidence, lines, 95% confidence interval, diamond plot, overall results of the included trials.
Incidence of ALT elevation
Nine studies reported data on all-grade ALT elevation, and were analyzed for the incidence of ALT elevation. A total of 895 patients were available for analysis. Incidences ranged from 22.9% to 52.4% with the highest incidence observed in a phase II trials in patients with metastatic breast cancer [Citation5,Citation9,Citation18], and the lowest in a phase II trial in patients with metastatic NSCLC [Citation13]. Among the patients who were administered pazopanib, the summary incidence of all-grade ALT elevation was 41.4% (95% CI 34.1–49.0%), using a random-effects model (Q = 32.357, I2 = 75.276, p < 0.001).
All 10 trials reported incidences of high-grade ALT elevation, with the highest incidence (12.1%) observed in a phase III trial in patients with RCC [Citation5], and the lowest incidence (2.7%) seen in a phase II trial in patients with recurrent cervical cancer [Citation8]. Using a fixed-effects model (Q = 10.712, I2 = 15.984, p = 0.296), the summary incidence of high-grade ALT elevation was 9.4% (95% CI 7.8–11.4%, ).
Variation of transaminase elevation with tumor types
We hypothesized that the risk of pazopanib-associated liver toxicity may be affected by underlying tumor types; we particularly examined RCC versus non-RCC tumors, since a large number of patients had RCC in this meta-analysis. In order to explore the heterogeneity of all-grade AST and ALT elevation, we calculated their incidences according to tumor types. Interestingly, there was a significant difference in the incidence of all-grade AST elevation between the 515 RCC patients [Citation5,Citation18] and the 345 non-RCC patients [Citation7–9,Citation11,Citation12,Citation16] (5.3% vs. 3.3%, p = 0.002). Similarly, the same 515 RCC patients [Citation5,Citation18] also demonstrated a significantly higher incidence in all-grade ALT elevation compared to the 380 non-RCC patients [Citation7–9,Citation11–13,Citation16] (5.2% vs. 3.7%, p = 0.004). As there is heterogeneity in non-RCC patients, we also compared RCC with sarcoma patients only. We did not find any significant difference.
However, there was no significant difference in the incidence of high-grade AST elevation between RCC patients [Citation5,Citation18] and non-RCC patients [Citation7–9,Citation11,Citation12,Citation16,Citation17] (7.4% vs. 6.3%, p = 0.34). There was also no significant difference in the incidence of high-grade ALT elevation between the 515 RCC patients [Citation5,Citation18] and 619 non-RCC patients [Citation7–9, Citation11–13,Citation16,Citation17] (10.9% vs. 7.9%, p = 0.08) analyzed.
Relative risk of transaminase elevation
To assess the specific role of pazopanib on the development of severe transaminase elevation, and to exclude the influence of confounding factors such as underlying malignancy and history of other therapeutic interventions, we determined RRs of all-grade and high-grade AST and ALT elevation with pazopanib when compared to controls. A meta- analysis of RR was performed on two RCTs in which the incidences of such toxicity were compared between pazopanib and placebo (). Using a fixed-effects model (Q = 0.465, I2 < 0.001, p = 0.495), the summary RR of high-grade AST elevation was determined to be 6.56 (95% CI 2.04–21.08, p = 0.002) in comparison with placebo, suggesting a significantly increased risk with pazopanib. The summary RR of high-grade ALT elevation was determined to be 4.33 (95% CI 1.88–10.0, p = 0.001) using a fixed-effects model (Q = 1.470, I2 = 31.987, p = 0.225), again suggesting a significantly increased risk. Taken together, pazopanib significantly increased the risk of high-grade liver toxicity more than six-fold for AST elevation and four-fold for ALT elevation in comparison with placebo. In addition, the summary RRs for all-grade AST or ALT elevation were significantly elevated ().
Figure 3. Relative risk of high-grade AST, ALT, and bilirubin elevation associated with pazopanib versus placebo. Summary relative risks were calculated using a fixed-effects model for high-grade AST (A), ALT (B), and bilirubin elevation (C). RR for each study is displayed numerically on the left and graphically on the right. Total events and sample sizes are also displayed for each study. For study name, the first author's name was used to represent each trial. For each trial: filled-in square, incidence, lines, 95% confidence interval, diamond plot, overall results of the included trials.
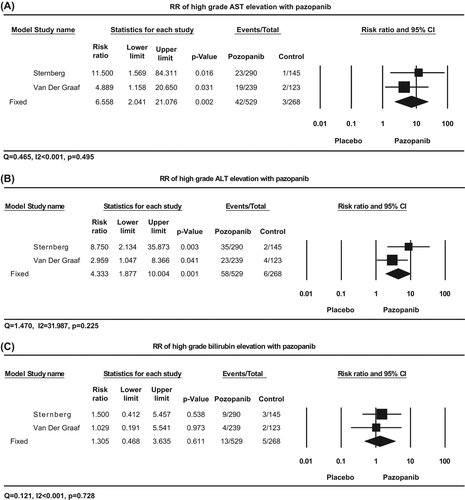
Table II. Incidence and relative risk of liver toxicity with pazopanib.
Incidence and risk of bilirubin elevation
Six trials included data pertaining to all-grade bilirubin elevation from a total of 860 patients with solid tumor types. Incidences ranged from 2.7% to 38.0%. Among the patients who were administered pazopanib, the summary incidence of all-grade bilirubin elevation was 24.8% (95% CI 16.6–35.3%), using a random-effects model (Q = 8.081, I2 = 25.751, p = 0.232).
Seven trials reported incidences of high-grade bilirubin elevation, ranging from 0.7% to 6.3%. Using a fixed-effects model (Q = 8.081, I2 = 25.751, p = 0.232), the summary incidence of high-grade bilirubin elevation was 3.4% (95% CI 2.3–5.0%, ).
A meta-analysis of RR was performed on two RCTs in which the incidences of high-grade bilirubin elevation were compared between pazopanib and placebo (). Using a fixed-effects model (Q = 0.121, I2 < 0.001, p = 0.728), the summary RR of high-grade bilirubin elevation was determined to be 1.31 (95% CI 0.47–3.64, p = 0.61) in comparison with placebo, suggesting a non-significantly increased risk with pazopanib (). The RR for all-grade bilirubin elevation was determined from the data of one trial [Citation5], and showed a significantly increased risk associated with pazopanib in comparison with placebo (RR 3.40, 95% CI 2.05–5.63; p < 0.001).
Risk of fatal adverse events and fatal hepatotoxicity
Among a total of 797 patients (pazopanib 529, placebo 268) from two trials with available data [Citation5,Citation6], there were a total of 20 and 10 FAEs for pazopanib and placebo, respectively. The summary incidence and RR of fatal toxicity with pazopanib was 3.8% (95% CI 2.5–2.7%) and 0.98 (95% CI 0.46–2.11, p = 0.97), respectively.
Of these fatal events, two were attributed to abnormal liver function [Citation5]. A review of the manufacturer's FDA briefing document addendum also describes a fatal outcome in a patient with synovial sarcoma as a result of rapidly progressing liver failure that was deemed secondary to treatment [Citation19]. Overall, the incidence and RR of fatal hepatoxicity with pazopanib was 0.7% (95% CI 0.2–2.7%) and 2.51 (95% CI 0.12–51.91, p = 0.55), respectively.
Publication bias
No publication bias was detected for high-grade AST elevation (Begg's test, two-tailed: p = 0.348) and high-grade ALT elevation (Begg's test, two-tailed: p = 0.210).
Discussion
Our study has demonstrated that pazopanib is associated with a significantly increased risk of liver toxicity based on the meta-analysis of 10 clinical trials including 1478 patients with a variety of solid tumors. The summary incidences of all-grade AST, ALT, and bilirubin elevation were 39.6% (95% CI 31.2–48.6%), 41.4% (95% CI 34.1–49.0%), and 24.8% (95% CI 16.3–35.3%), respectively with 6.9% (95% CI 5.5–8.6%), 9.4% (95% CI 7.8–11.4%), and 3.4% (2.4–5.0%) being high-grade, respectively. In comparison with placebo, pazopanib significantly increased the risk of high-grade AST elevation (RR 6.56, 95% CI 2.04–21.07, p = 0.002) and ALT elevation (RR 4.33, 95% CI 1.88–10.0, p = 0.001). Therefore, the risk of serious liver toxicity is considerable in cancer patients. However, pazopanib did not significantly increase the risk of high-grade bilirubin elevation (RR 1.31, 95% CI 0.47–3.64) and fatal hepatotoxicity (RR 2.51, 95% CI 0.12–51.91, p = 0.55). This study will help physicians and patients to fully understand the risk of drug-induced liver injury (DILI) with pazopanib therapy in cancer patients. With the recent approval of its application in soft tissue sarcoma in addition to the previously approved use in advanced RCC, pazopanib will be increasingly used in routine cancer therapy as well as clinical trials. Awareness of such risks and close monitoring could permit early appropriate intervention to reduce morbidity and mortality associated with liver damage.
Drug-induced liver injury remains the most common AE resulting in product withdrawals and study terminations. Several theories regarding its pathogenesis have been postulated including immune-mediated toxicity, mitochondrial dysfunction, variations in host metabolic response, or less commonly, direct toxicity to hepatocytes [Citation22,Citation23]. The role of immune-mediated toxicity is supported by recent pharmacogenetic studies which demonstrated strong association between human leukocytic antigen (HLA) polymorphisms and drug-induced liver injury [Citation24]. So far, there is no evidence to support an association between HLA alleles and pazopanib-related ALT elevations [Citation25]. Pazopanib may directly affect metabolism in the liver. It is metabolized by CYP3A4 with a minor contribution from CYP 1A4 and CYP2C8, and inhibits UGT1A1, which glucuronidate bilirubin for elimination. A study has identified a significant association of UGT1A1 Gilbert's polymorphism with pazopanib-induced bilirubin elevation in 236 patients with RCC [Citation26].
Pazopanib may also cause liver toxicity due to its on-target effect. Of the several targets inhibited by pazopanib, VEGFR-1 and VEGFR-2 are key players in liver organogenesis and regeneration. VEGFR-1 is expressed on hepatocytes during regeneration, and may mediate an angiogenesis-independent endothelial protection of hepatocytes. In a study involving mice exposed to a hepatotoxin carbon tetrachloride (CCl4), activation of liver sinusoidal endothelial cells through VEGFR-1 yielded secretion of hepatocyte growth factor (HGF) and interleukin 6 (IL-6) [Citation27]. Additionally, VEGF-A production is concomitantly increased in both hepatocytes and non-parenchymal cells following liver damage. These up-regulated factors are believed to promote hepatocyte proliferation and protect hepatocytes against toxin-induced injury [Citation28]. Thus, it is conceivable that pazopanib-related inhibition of VEGFR-1 may render hepatocytes less capable of withstanding damage resulting from hepatotoxins [Citation29]. Interestingly, a retrospective study showed an association between germline variants in the HFE gene and liver injury in pazopanib-treated white patients with RCC [Citation25]. HFE, the hemochromatosis gene, encodes a membrane protein that is intricately involved in iron homeostasis. Mutations in this gene result in abnormal iron metabolism and/or storage with resultant oxidative stress. Available literature suggests that HFE and VEGFR-2 have many hypoxia-induced transcriptional regulators in common, and the inhibition of various VEGF signals may reduce induction of HFE [Citation30]. Hence, treatment with VEGFR inhibitor pazopanib may compromise HFE function varied with its polymorphism, and predispose some patients to hepatocellular injury and subsequent ALT elevations.
Liver toxicity is an emerging issue in the use of angiogenesis inhibitors. In addition to pazopanib, liver toxicity has also been associated with the use of other VEGFR inhibitors such as sunitinib, sorafenib and axitinib (). Cases of acute hepatic failure following monotherapy with sunitinib or sorafenib have also been reported [Citation31,Citation32]. It is currently not clear whether these agents have different liver toxicity profile. However, it appears that pazopanib is associated with a higher incidence of high-grade liver toxicity when compared to the other agents, as shown in . This is also consistent with the result from a recent phase III trial in which pazopanib was directly compared to sunitinib in 1002 RCC patients (COMPAZ), showing ALT elevation is significantly higher with pazopanib than sunitinib (31% vs. 18%) [Citation33]. The reasons for this difference may be a product of different receptor affinities, liver metabolism, and target difference even though their targets are similar.
Table III. Risk of liver toxicity with VEGFR tyrosine kinase inhibitors.
Our study also demonstrated that the risk of transaminase elevation associated with pazopanib varies among patients with different tumor types. Overall, patients with RCC demonstrated an increased risk of liver toxicity compared to non-RCC patients. A possible explanation for this finding may be related to reduced renal function associated with prior nephrectomy in RCC patients. In the RCC trials included in our analysis, approximately 90% of patients had a prior nephrectomy. However, given that pazopanib is metabolized primarily by the liver and eliminated in the feces, abnormal renal function alone may not account for the difference. Moreover, renal excretion accounts for less than 4% of pazopanib's clearance [Citation34]. Another possible explanation may be related to prior cytokine treatment in RCC, which may predispose these patients to liver damage associated with pazopanib.
Concern for liver damage in patients treated with pazopanib resulted in severe and fatal hepatotoxicity being listed as a black box warning by FDA. Although severe liver failure is indeed rare, the package insert notes two fatal outcomes from initial studies attributed to disease progression and hepatic failure. Four patients in the pazopanib arm had FAEs deemed to be related to study treatment including two with abnormal hepatic function [Citation5,Citation18]. Bearing the limitations of small numbers, our analysis showed that pazopanib was associated with a low risk of fatal hepatotoxicity, and did not significantly increase the risk of fatal hepatic AEs (incidence: 0.7%, 95% CI 0.2–2.7%; RR: 2.51, 95% CI 0.12–51.91, p = 0.552), which is somewhat reassuring. This may help patients and physicians to appropriately understand the risk of fatal toxicity associated with pazopanib.
Current recommendations for the monitoring and management of pazopanib-related liver toxicity are mostly based on the experiences from clinical trials. Pre-treatment laboratory workup should include baseline liver function tests followed by transaminase monitoring every four weeks for at least the first four months [Citation34]. However, others have suggested that liver-function test monitoring every two weeks during the first eight weeks of pazopanib therapy based on some case series [Citation35]. The manufacturer has recommended a dose adjustment for baseline moderate hepatic impairment. It is currently contraindicated in patients with severe hepatic impairment. The majority of susceptible patients will experience liver enzyme elevations in the first few months of drug exposure with return to baseline levels upon treatment interruption. In most studies used in our analysis, dose interruptions, adjustments, or discontinuations were made in response to raised transaminase levels. For Grade 3 or higher aminotransferase elevations, pazopanib was typically held until return to pre- treatment levels. Pazopanib was then resumed with a decrease in dose in increments of 200 mg.
Our meta-analysis has several limitations. Firstly, these studies were conducted at various institutions by distinct investigators and may have potential bias in reported incidences or specification of laboratory abnormalities. The reported incidence of all-grade transaminase elevation had significant heterogeneity among the included studies. Nevertheless, we have minimized its influence by using a random-effects model to calculate the overall incidence. Secondly, enrolled patients typically had adequate organ function and may not reflect the general patient population seen in clinical practice. Thirdly, this is a meta-analysis at study level; therefore confounding variables at the patient level cannot be assessed properly and incorporated into the analysis. Finally, even though our analysis showed there was no statistically significant difference in incidence of fatal hepatic toxicity between pazopanib and placebo, the conclusion may be limited by a small number of studies with available data for analysis.
In summary, our study has quantified the overall risk of liver toxicity associated with pazopanib in cancer patients, and demonstrated that pazopanib significantly increased the risk, but not fatal hepatotoxicity. It is important for physicians and patients to appropriately recognize the risks as well as the benefits associated with pazopanib treatment with regular monitoring of serum transaminases. It is important to identify patients at high risk for liver toxicity. It was shown that pazopanib trough concentration, age of patient, and baseline ALT may be important factors in predicting ALT elevations in pazopanib treated mRCC patients [Citation36]. Future studies are recommended to investigate risk reduction and to optimize pazopanib-based therapy.
Declaration of interest: The authors alone are responsible for the content and writing of the paper. T. K. Choueiri is on the scientific advisory board of GSK, Pfizer, Aveo, Genentech, Novartis and Bayer/Onyx. Dr Wu has received honoraria from Onyx Pharmaceuticals, Pfizer, Jansen, and Novartis, and is a speaker for Onyx, Pfizer, Jansen, and Novartis and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27.
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29 (6 Suppl 16):15–8.
- Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol 2000;50: 63–70.
- Cowey CL, Sonpavde G, Hutson TE. New advancements and developments in treatment of renal cell carcinoma: Focus on pazopanib. Onco Targets Ther 2010;3:147–55.
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8.
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379: 1879–86.
- Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schoffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–32.
- Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol 2010;28:3562–9.
- Taylor SK, Chia S, Dent S, Clemons M, Agulnik M, Grenci P, et al. A phase II study of pazopanib in patients with recurrent or metastatic invasive breast carcinoma: A trial of the Princess Margaret Hospital phase II consortium. Oncologist 2010;15:810–8.
- GlaxoSmithKline. Votrient [package insert]. Research Triangle Park, NC, 2012.
- Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 2010;12:855–61.
- Friedlander M, Hancock KC, Rischin D, Messing MJ, Stringer CA, Matthys GM, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol 2010;119:32–7.
- Altorki N, Lane ME, Bauer T, Lee PC, Guarino MJ, Pass H, et al. Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non-small-cell lung cancer. J Clin Oncol 2010;28:3131–7.
- Lim WT, Thng C, Toh CK, Chowbay B, Goh BC, Leong S, et al. A phase II study of GW786034 (pazopanib) in Asian patients with recurrent/metastatic undifferentiated nasopharyngeal carcinoma. ASCO Meeting Abstracts. 2010;28 (15 Suppl):5556.
- Phan AT, Yao JC, Fogelman DR, Hess KR, Ng CS, Bullock SA, et al. A prospective, multi-institutional phase II study of GW786034 (pazopanib) and depot octreotide (sandostatin LAR) in advanced low-grade neuroendocrine carcinoma (LGNEC). ASCO Meeting Abstracts. 2010 June 14. 2010;28 (15 Suppl):4001.
- Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol 2010;11:962–72.
- Van Der Graaf TA. PALETTE: A randomized, double-blind, phase III trial of pazopanib versus placebo in patients (pts) with soft-tissue sarcoma (STS) whose disease has progressed during or following prior chemotherapy—An EORTC STBSG Global Network Study (EORTC 62072). Chicago: ASCO; 2011.
- Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 2010;28:475–80.
- Committee FODA. Votrient (Pazopanib) Tablets: For treatment of patients with advanced renal cell carcinoma. Addendum to GSK briefing document for October 5, 2009. 2009 [cited 2011 Feb 21]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM184009.pdf
- Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA 2008;300:2277–85.
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 2007;49:186–93.
- Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut 2009;58:1555–64.
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med 1995;333:1118–27.
- Andrade RJ, Robles M, Ulzurrun E, Lucena MI. Drug-induced liver injury: Insights from genetic studies. Pharmacogenomics 2009;10:1467–87.
- Xu CF, Reck BH, Goodman VL, Xue Z, Huang L, Barnes MR, et al. Association of the hemochromatosis gene with pazopanib-induced transaminase elevation in renal cell carcinoma. J Hepatol 2011;54:1237–43.
- Xu CF, Reck BH, Xue Z, Huang L, Baker KL, Chen M, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer 2010;102:1371–7.
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, et al. Angiogenesis-independent endothelial protection of liver: Role of VEGFR-1. Science 2003;299: 890–3.
- Dufour J-F, Clavien P-A. Signaling pathways in liver diseases, 2nd ed. New York: Springer; 2009.
- Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology 2011;140:1410–26.
- Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol 2007;144:761–8.
- Taran A, Ignatov A, Smith B, Costa SD, Bischoff J. Acute hepatic failure following monotherapy with sunitinib for ovarian cancer. Cancer Chemother Pharmacol 2009;63:971–2.
- Schramm C, Schuch G, Lohse AW. Sorafenib-induced liver failure. Am J Gastroenterol 2008;103:2162–3.
- Motzer Rea. COMParing the efficacy, sAfety and toleRability of paZopanib vs. sunitinib (COMPAZ). The ESMO 2012 Congress of the European Society for Medical Oncology; 2012. Vienna, Austria: ESMO; 2012.
- GlaxoSmithKline. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
- Klempner SJ, Choueiri TK, Yee E, Doyle LA, Schuppan D, Atkins MB. Severe pazopanib-induced hepatotoxicity: Clinical and histologic course in two patients. J Clin Oncol 2012;30:e264–8.
- Pandite L, Goodman V, Botbyl J, Suttle AB, Gauvin J, Dar M. Trough PK concentration, age of patient, and baseline ALT are important factors in predicting ALT elevations in pazopanib treated mRCC patients. Meeting Abstracts. Lugano: European Society for Medical Oncology (ESMO); 2010.
- Pfizer. Sutent [package insert]. New York, NY: Pfizer: 2012.
- Pfizer. Inlyta (Axitinib) package insert. New York, NY: Pfizer: 2012.