Abstract
Purpose. The aim of the study was to improve computed tomography (CT)-based high-risk clinical target volume (HR CTV) delineation protocols for cervix cancer patients, in settings without any access to magnetic resonance imaging (MRI) at the time of brachytherapy. Therefore the value of a systematic integration of comprehensive three-dimensional (3D) documentation of repetitive gynecological examination for CT-based HR CTV delineation protocols, in addition to information from FIGO staging, was investigated. In addition to a comparison between reference MRI contours and two different CT-based contouring methods (using complementary information from FIGO staging with or without additional 3D clinical drawings), the use of standardized uterine heights was also investigated. Material and methods. Thirty-five cervix cancer patients with CT- and MR-images and 3D clinical drawings at time of diagnosis and brachytherapy were included. HR CTVstage was based on CT information and FIGO stage. HR CTVstage + 3Dclin was contoured on CT using FIGO stage and 3D clinical drawing. Standardized HR CTV heights were: 1/1, 2/3 and 1/2 of uterine height. MRI-based HR CTV was delineated independently. Resulting widths, thicknesses, heights, and volumes of HR CTVstage, HR CTVstage + 3Dclin and MRI-based HR CTV contours were compared. Results. The overall normalized volume ratios (mean± SD of CT/MRIref volume) of HR CTVstage and HR stage + 3Dclin were 2.6 (± 0.6) and 2.1 (± 0.4) for 1/1 and 2.3 (± 0.5) and 1.8 (± 0.4), for 2/3, and 1.9 (± 0.5) and 1.5 (± 0.3), for 1/2 of uterine height. The mean normalized widths were 1.5 ± 0.2 and 1.2 ± 0.2 for HR CTVstage and HR CTVstage + 3Dclin, respectively (p < 0.05). The mean normalized heights for HR CTVstage and HR CTVstage + 3Dclin were both 1.7 ± 0.4 for 1/1 (p < 0.05.), 1.3 ± 0.3 for 2/3 (p < 0.05) and 1.1 ± 0.3 for 1/2 of uterine height. Conclusion. CT-based HR CTV contouring based on FIGO stage alone leads to large overestimation of width and volume. Target delineation accuracy can systematically improve through incorporation of additional information from comprehensive 3D documentation of repetitive gynecological examination in the contouring protocol, and thus help to improve the accuracy of dose optimization in settings with limited access to imaging facilities at the time of brachytherapy. If CT information is only available, minimum 2/3 of uterine height may be a good surrogate for the height of HR CTV.
Cervical cancer is the third most common female cancer in women and is the second highest cause of female cancer mortality worldwide [Citation1–3]. The invasive cervical cancer is highly prevalent in developing countries, where it accounts for 15% of female cancers [Citation4]. Especially in these countries it is difficult to apply the most modern techniques for brachytherapy (BT) planning.
Three-dimensional (3D) image-based BT planning in cervical carcinoma started to increase with the availability of computed tomography (CT)/magnetic resonance imaging (MRI)-compatible applicators, treatment planning systems, and guidelines [Citation5–7], which allow conformal planning and adaptation of dose to target volumes and organs at risk (OARs) [Citation8,Citation9]. Recent reports indicate that image-guided BT may improve local control and decrease treatment-related morbidity [Citation10].
MRI is considered the best imaging modality for assessing cervical tumor extent compared with CT [Citation11,Citation12]. For parametrial invasion and advanced disease there is reasoned indication that MRI assessment may be significantly better for staging of cervical carcinoma compared to clinical assessment [Citation13]. The brachytherapy group of the European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) provided recommendations for MRI-guided BT in cervix cancer including definitions for: gross tumor volume (GTV), high-risk clinical target volume (HR CTV) and intermediate-risk CTV (IR CTV) [Citation6,Citation7].
Assessment of cervical cancer on CT is limited, as tumor dimensions and invasion of uterine corpus or parametria may not be detected accurately. In a multicentric study by ACRIN/GOG, MRI proved significantly better than CT for tumor visualization and detection of parametrial invasion of cervical cancer [Citation14]. CT allows limited quantitative estimation of tumor regression during radiotherapy and cannot distinguish between normal tissue and residual disease [Citation15]. Some studies reported that CT-based contouring is feasible for OARs but may overestimate the target volume as recognition of tumor and surrounding normal tissue is often not possible [Citation16,Citation17]. There are currently no widely agreed upon guidelines for CT-guided BT especially if MRI is not available at diagnosis or at time of BT [Citation16].
However, there is a long tradition of clinical gynecologic examination (CGE) in cervical cancer, which represents the gold standard of FIGO stage definition [Citation18]. Clinical findings can be documented on diagrams with schematic drawings in all body orientations and a speculum view [Citation6,Citation19]. According to MRI-CT pathologic studies, the value of CGE seems to be comparable to MRI regarding stage allocation [Citation20]. For these reasons, CGE has been highlighted in the GEC-ESTRO Recommendations on MRI-guided BT as a complementary tool for stage allocation and tumor spread in clinically accessible regions (cervix, vagina, parametria) [Citation7,Citation12].
The aim of our study was to evaluate different approaches of CT-based HR CTV delineation, for clinical settings where no access to MRI facilities is available for BT treatment planning. We investigated if and to what extent a systematic inclusion of precise 3D documentation of CGE in a purely CT-based delineation protocol, compared to FIGO stage information alone [Citation18], can improve CT-based target contouring. As tumor length cannot be assessed on CT, the value of using different standardized uterine heights was investigated. For quality assessment of the CT-based HR CTV contours, MRI-based HR CTV delineation was used as a reference.
Material and methods
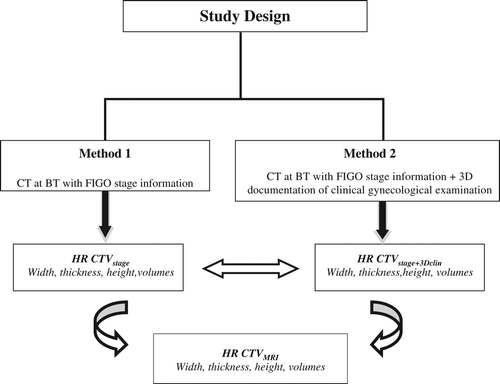
This study was designed to compare CT-based HR CTV delineation using information from FIGO stage with or without 3D documentation of CGE with a reference of MRI-based HR CTV delineation regarding total volume, width, height and thickness of HR CTV ().
Patients and treatment
Thirty-five patients with cervix cancer were retrospectively selected from our overall patient cohort (n = 165, 2001–2008) on the basis of availability of both CT and MRI with applicator in place and full 3D documentation (diagram drawings) representing CGE [Citation10].
All patients had biopsy-proven cervical cancer [FIGO stages IB (8), IIB (18), and III (9)]. They received definitive chemoradiotherapy (45–50 Gy EBRT) with curative intent. All patients were treated with 4 fractions of MRI-guided BT to achieve a total D90 for HR CTV of ≥ 85 Gy EQD2 (α/β = 10 Gy). CT/MRI-compatible tandem-ring applicators with (17) or without (18) interstitial needles were used (Nucletron Systems, Veenendaal, The Netherlands). Institutional imaging protocols for MRI and CT acquisition with applicator in place have been previously described [Citation15,Citation16].
Clinical examination and 3D documentation with diagram drawing
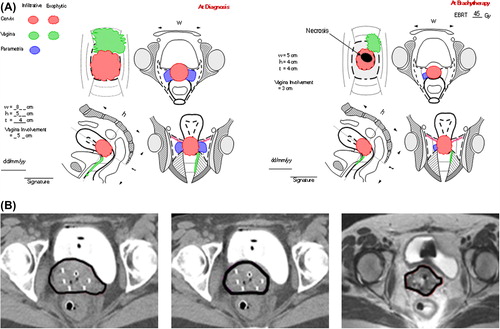
The CGE was performed by an expert radiation oncologist and included vaginal inspection, palpation and transrectal examination. Accurate documentation of clinical findings was done by drawing on a specific standard diagram () developed within the framework of the Gyn GEC-ESTRO recommendations [Citation6] and further adapted for the EMBRACE study (available at https://www.embracestudy.dk).
Figure 2. A) 3D clinical drawing of CGE for patient with large tumor in whole cervix with bilateral parametrial disease and vaginal infiltration along anterior and left wall reaching the lower third of vagina (FIGO stage IIIA) at diagnosis, Residual tumor after radiochemotherapy at time of BT was still in whole cervix with a central necrosis extending up to the right mid-parametrium and in the anterior-left upper third of vaginal wall. B) HR CTV delineation for the same patient on transverse CT section through the low cervix region with tandem applicator and interstitial needles: CT-based HR CTVstage, CT-based HRCTVstage + 3Dclin and MRI-based HR CTVMRI.
MRI and HR CTV contouring
T2-weighted MRI studies after BT applicator placement were generated in 5 mm slice intervals. The HR CTVMRI was contoured according to the Gyn GEC-ESTRO recommendations [Citation6,Citation7] using PLATO/Oncentra Masterplan (OMP) workstations (Nucletron, Veenendaal, The Netherlands), by an expert gynecological radiation oncologist (RP, AS).
CT imaging and HR CTV contouring
The CT images at time of BT with applicator in place were generated in 4 mm slice intervals. The CT scanner is a conventional multislice CT scanner (Somatom plus S, Siemens, Erlangen, Germany) [Citation6]. CT scans were performed using contrast agents for bladder only. Contouring was done without knowledge of MRI findings by another expert radiation oncologist (NH) on an OMP workstation (Nucletron, Veenendaal, The Netherlands). HR CTVstage was delineated using information on FIGO stage only. HR CTVstage + 3Dclin was delineated by the same observer, taking additionally into account 3D documentation of CGE, as depicted on the clinical diagrams ().
As cranial tumor extension cannot be accurately assessed by FIGO, CGE or CT, in advanced disease, a standardized approach was chosen. Uterine height was measured on CT (sagittal plane) from the most cranial part of uterine fundus to most caudal slice of cervical tissue (at ring level). The height of HR CTVstage and HR CTVstage + 3Dclin were contoured using three different standardized uterine heights: 1/1, 2/3 and 1/2 of the uterus. These heights were investigated to assess which one would correspond best to the height as individually assessed from MRI (height of HR CTVMRI) . For each patient six CT-based HR CTVs were designed: 1) HR CTVstage with 1/1, 2/3, 1/2 uterine height; and 2) HR CTVstage + 3Dclin with 1/1, 2/3 and 1/2 uterine height.
HR CTVstage volumes were contoured on CT based on information of FIGO stage [Citation18] and preliminary CT-based contouring guidelines [Citation7]. For stage IB, HR CTVstage encompassed the whole cervix as seen on CT. In FIGO stage IIB, HR CTVstage included the entire cervix and parametria on both sides irrespective of the CT findings (as accurate distinction between tumor and parametrium and invasion of parametria may not always be detected accurately on CT). For stage IIIA, HR CTVstage included the entire cervix, and the whole parametria if involved (). In stage IIIB the entire cervix and both parametria to the pelvic side wall were contoured, respecting anatomical boundaries.
HR CTVstage + 3Dclin volumes were contoured on CT with integration of information from 3D documentation of precise CGE at the time of BT. For stage IB the entire cervix seen on CT with dimensions comparable to CGE was contoured. In IIB, HR CTVstage + 3Dclin encompassed cervix and residual parametrial extension according to dimensions and location identified by CGE. For IIIA (), HR CTVstage + 3Dclin included cervix, involved parametria and vagina at the time of BT. In IIIB, HR CTVstage + 3Dclin included the entire cervix, and residual parametrial disease as described in the clinical drawings ().
Evaluation
Three-dimensional parameters, i.e. maximum height, thickness, and width, were measured for each HR CTV. The height of CT-based HR CTV indicates the cranio-caudal diameter assessable on mid-sagittal view along the uterine axis. The thickness was measured as the largest anteroposterior diameter on axial view. The width is the largest transverse diameter, measured on axial view. The volumes of all HR CTVs calculated by the treatment planning system were recorded for each patient.
The resulting 3D parameters of HR CTVstage and HR CTVstage + 3Dclin were compared with each other, and also with HR CTVMRI (). Width, height, and volume parameters for HR CTVstage and HR CTVstage + 3Dclin were normalized to corresponding HR CTVMRI reference values, and resulting CT/MRI ratios were reported, e.g. volume ratio VRx = Vx / VHR CTV MRI, where x stands for HR CTVstage or HR CTVstage + 3Dclin. For comparison between the different HR CTV volume parameters a paired Wilcoxon-rank test was performed. P-values < 0.05 were considered significant.
Results
Thirty-five patients with cervical cancer were evaluated. The mean values and standard deviations of height, width, thickness and volume of HR CTVstage. HR CTVstage + 3Dclin and HR CTVMRI are listed in . All parameters are reported as mean ± 1 standard deviation (SD). Statistically significant differences between the volumes of HR CTVstage and HR CTVstage + 3Dclin (each with 1/1, 2/3, 1/2 of uterine height) were found (p < 0.05). The volumes of HR CTVstage and HR CTVstage + 3Dclin were significantly larger (p < 0.05) than the volumes of HR CTVMRI irrespective of chosen uterine height (). The contouring of 1/1 uterine height for HR CTVstage and HR CTVstage + 3Dclin resulted in significantly larger volumes. The mean normalized volumes (CT/MR volume ratio) of HR CTVstage and HR CTVstage + 3Dclin were 2.6 ± 0.6 and 2.1 ± 0.4. The place and extent of parametrial infiltration was not detectable on CT in more than 60% of cases.
Figure 3. Significant decrease of the volume ratios (VR) of HR CTV by adding of CGE diagrams, for all standardized uterine lengths (1/1, 2/3, 1/2) used in the study. VRstage is the volume ratio of HR CTVstage/HR CTVMRI and VRstage + 3Dclin is the volume ratio of CT-based HR CTVstage + 3Dclin/HR CTVMRI.
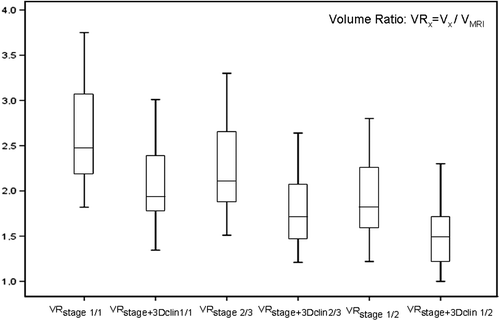
Table I. Mean, standard deviations and ranges of volume, height, width, and thickness of HR CTVstage, HR CTVstage + 3Dclin and HR CTVMRI.
The widths of the CT-based HR CTVs were larger than the widths of HR CTVMRI. The differences between the normalized widths of both HR CTVstage (1.5 ± 0.2) and HR CTVstage + 3Dclin (1.2 ± 0.2), and HR CTVMRI (1) were statistically significant (), as well as the difference between the mean normalized widths of HR CTVstage and HR CTVstage + 3Dclin. The overestimation of the widths of HR CTV volumes contoured on CT was decreased by adding CGE information (). No statistically significant differences were noticed in regard to thickness ().
Figure 4. Significant decrease of the width ratio (WR) of HR CTV by adding of CGE diagrams. WRstage is the width ratio of CT-based HR CTVstage/HR CTVMRI and WRstage + 3Dclin is the width ratio of CT-based HR CTVstage + 3Dclin/HR CTVMRI.
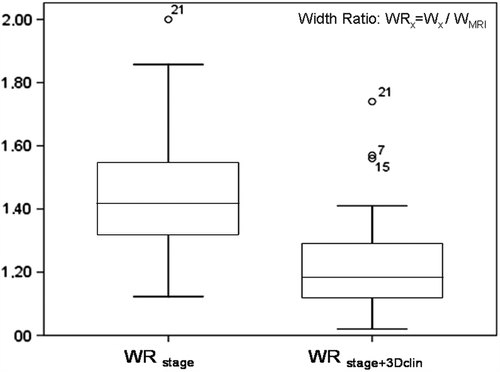
Table II. Mean and standard deviations of the CT/MRI width ratios (WRx = Wx/WMRI) thickness ratios (TRx = Tx/TMRI) for all HR CTVstage and HR CTVstage + 3Dclin, and heights ratios (HRx = Hx/HMRI) for all standard uterine heights used for both CT-based contour types, grouped by different FIGO stages.
For patients with stage IB (8/35), the mean height ratio (CT height/MRI height) of HR CTVstage and HR CTVstage + 3Dclin including 1/2 uterine height was 1.1 ± 0.1. For patients with stage IIB and III (27/35), heights of CT-based HR CTVs including 1/2 uterine height were smaller than the heights of HR CTVMRI in 10 patients (six stage IIB and four stage III). The height of CT-based HR CTV including 2/3 uterine height was slightly smaller than the height of HR CTVMRI in three patients (two stage IIB and one stage III). These three patients had extensive intrauterine tumor infiltration at time of BT and the whole intrauterine tandem lengths had to be loaded to achieve sufficient dosimetric coverage of the original HR CTVMRI.
Discussion
In order to evaluate the potential improvement of a fully CT-based delineation protocol, for settings without access to MRI at the time of brachytherapy, the use of information based on FIGO stage with/without adding precise documentation of gynecological findings was investigated in this study. The quality of the CT-based HR CTV contours was assessed by comparison with the gold standard, i.e. MRI-based target delineation. The results indicate a clear advantage of CT contouring when taking into account precise information from CGE. The volumes of HR CTVstage were statistically significantly larger than those of HR CTVstage + 3Dclin, as contours based on FIGO stage alone lead to a large overestimation, especially of target width. The importance of CGE became most evident in width assessment and was not very pronounced for HR CTV thickness. The place and extent of parametrial infiltration was not detectable on CT in more than 60% of cases. The protocol had foreseen delineation of the whole parametria in case of FIGO stage information alone. This conservative approach provides upper limits for the overestimation of CT-based HR CTV volumes in case of very limited additional information.
Our study showed that HR CTVstage and HR CTVstage + 3Dclin volumes based on standard heights are significantly larger than HR CTVMRI volume, overestimating mainly the width and using standard heights. These findings are in agreement with other reports [Citation16,Citation17] and are due to the inferior soft tissue contrast of CT which does not allow identification of macroscopic tumor. The comprehensive information of disease spread based on clinical examination may contribute to reduce the inaccurate CT-based volume determination (in particular in width). This is shown by the present study by an improved conformity, through width and volume reduction, when comparing contouring based on gynecologic information with FIGO stage information only. CT-based HR CTV contouring should always be based on CGE [documented on a specific diagram ()]. This procedure is recommended by the GEC ESTRO even when MRI is available [Citation6,Citation7,Citation12], and also formed the basis of the CT-MRI study by Viswanathan et al. [Citation16]. Additional improvement most likely would be gained if the same person doing the gynecological examination is also doing the contouring, which was not the case for this study.
Several studies compared CT-guided BT with conventional BT and demonstrated that CT-based planning is superior to conventional planning, improving conformity of target coverage [Citation21]. Shin et al. [Citation21] used pre-treatment MRI to detect evidence of intrauterine tumor extension. The macroscopic residual cervical tumor was delineated on CT images with information of CGE and pre-treatment MRI.
Our study tried additionally to assess the potential of using various standard uterine heights for CT-based HR CTV height determination. The height of the individual uterine tumor invasion could not be assessed in the study population, as neither CT nor CGE are capable of assessing uterine tumor invasion in cervix carcinoma. Therefore a standardized approach was investigated by contouring three different uterine heights (whole, 2/3 and 1/2 of uterus). Excessively large volumes were observed in patients with stage IB where such extensive contouring seems to be unnecessary. The very pronounced volume effect of choosing a standard height of 2/3 or 1/1 of the uterine corpus presents the major difference compared to results in a former study [Citation16]. In the latter, an assumed dome shaped cranial cervix border was contoured based on CT information.
As a conclusion of the present study, the CT-based contouring guidelines as suggested earlier by Viswanathan et al. [Citation16] have to be taken with caution, regarding the superior border of the HR CTV. According to these recommendations the cranial border of the HR CTV should be delineated at the level where uterine vessels first appear, or to a point where the uterine cavity appears. Then two slices of contour around the tandem superiorly were added to cover the cervical apex. However, it is clearly recognized in literature that for cranial tumor extension assessment, CT alone is insufficient [Citation14].
According to findings of the present study there may be a geographical miss in 10–35% of cases with advanced disease when defining the cranial border of HR CTV at 2/3 or 1/2 of the uterine height. Therefore, any tailoring of this border should be avoided, if only CT is available, except in cases of very limited disease. If MRI is not available, our recommendation is, consequently, to include most of the height along the uterine cavity into the HR CTV in advanced stages. A straightforward approach for CT-based treatment planning is to keep the full loading of intrauterine tandem length up to its tip as planning aim according to traditional practice.
The potential dosimetric benefit of our improved CT-based target structures has not been assessed in the present study. Overall, the reported DVH parameters for CT-based target structures are expected to underestimate systematically the dose to the corresponding MRI-based HR CTV, which is usually smaller according to present and former findings [Citation17], in particular in regard to height and width. The impact of volume on the D90 HR CTV for standard dose planning and dose prescription to point A was presented by Tanderup et al. [Citation22]. For example, increasing the HR CTV volume from 30 to 45 cm³ decreases the D90 by more than 20%.
Overall, our study shows that CT-based contouring can be significantly improved by careful integration of comprehensive CGE at diagnosis and at time of BT, in particular for appropriate width. The height of the HR CTV should be determined by applying standard heights. If only CT information is available (no MRI), the selection of at least two thirds of the uterine cavity as standard height will include most of the potential cranial tumor extension for advanced disease. The results presented here, are based on a medium-size cohort of patients who were evaluated retrospectively. Therefore, these findings need to be validated within a larger (multi-institutional) prospective trial. The use of most modern, contrast enhanced techniques might further improve the quality of CT-based contouring in general. As another alternative to CT and MRI, modern ultrasound techniques, adapted for treatment planning, might be used in the future [Citation23–25].
While MRI remains the gold standard for contouring HR CTV for 3D IGABT, CT-based delineation of HR CTV may be applied in situations with limited imaging resources. However, to arrive at a clinically acceptable accuracy such CT-contouring always has to be based on a comprehensive 3D documentation of repetitive gynecologic examination.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Austrian Science Fund (FWF), project L562-B19 and by the Austrian Federal Ministry of Economy, Family and Youth, and the National Foundation for Research, Technology and Development is gratefully acknowledged. The Department of Radiotherapy at the Medical University of Vienna receives financial and/or equipment support for research and educational purposes from Nucletron B.V. and Varian Medical Systems, Inc. C. Kirisits is a consultant to Nucletron, an Elekta company.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
- Kotaniemi-Talonen L, Malila N, Anttila A, Nieminen P, Hakama M. Intensified screening among high risk women within the organised screening programme for cervical cancer in Finland. Acta Oncol 2011;50:106–11.
- Lönnberg S, Leinonen M, Malila N, Anttila A. Validation of histological diagnoses in a national cervical screening register. Acta Oncol 2012;51:37–44.
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50.
- Nag S, Cardenes H, Chang S, Das IJ, Erickson B, Ibbott GS, et al. Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: Report from Image-Guided Brachytherapy Working Group. Int J Radiat Oncol Biol Phys 2004;60:1160–72.
- Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45.
- Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77.
- Pötter R, Kirisits C, Fidarova EF, Dimopoulos JC, Berger D, Tanderup K, et al. Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma. Acta Oncol 2008;47:1325–36.
- Wanderas AD, Sundset M, Langdal I, Danielsen S, Frykholm G, Marthinsen AB. Adaptive brachytherapy of cervical cancer, comparison of conventional point A and CT based individual treatment planning. Acta Oncol 2012;51:345–54.
- Pötter R, Georg P, Dimopoulos JC, Grimm M, Berger D, Nesvacil N, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23.
- Stenstedt K, Hellstrom AC, Fridsten S, Blomqvist L. Impact of MRI in the management and staging of cancer of the uterine cervix. Acta Oncol 2011;50:420–6.
- Dimopoulos J, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol 2012;103:113–22.
- Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur Radiol 2013;23:2005–18.
- Hricak H, Gatsonis C, Coakley FV, Snyder B, Reinhold C, Schwartz LH, et al. Early invasive cervical cancer: CT and MR imaging in preoperative evaluation – ACRIN/GOG comparative study of diagnostic performance and interobserver variability. Radiology 2007;245:491–8.
- Dimopoulos J, Schard G, Berger D, Lang S, Goldner G, Helbich T, et al. Systematic evaluation of MRI findings in different stages of treatment of cervical cancer: potential of MRI on delineation of target, pathoanatomic structures, and organs at risk. Int J Radiat Oncol Biol Phys 2006;64: 1380–8.
- Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 2007;68: 491–8.
- Eskander RN, Scanderbeg D, Saenz CC, Brown M, Yashar C. Comparison of computed tomography and magnetic resonance imaging in cervical cancer brachytherapy target and normal tissue contouring. Int J Gynecol Cancer 2010;20: 47–53.
- Pecorelli S. Revised FIGO staging of vulva, cervix and endometrium. FIGO committee on gynecological oncology. Int J Gynaecol Obstet 2009;105:103–4.
- Fletcher GH. Textbook of radiotherapy. Philadelphia, PA: Lea & Febiger 1980; 720–72.
- Mitchell DG, Snyder B, Coakley F, Reinhold C, Thomas G, Amendola M, et al. Early invasive cervical cancer: Tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol 2006;24:5687–94.
- Shin KH, Kim TH, Cho JK, Kim JY, Park SY, Park SY, et al. CT-guided intracavitary radiotherapy for cervical cancer: Comparison of conventional point A plan with clinical target volume-based three-dimensional plan using dose-volume parameters. Int J Radiat Oncol Biol Phys 2006;64:197–204.
- Tanderup K, Nielsen SK, Nyvang GB, Pedersen EM, Rohl L, Aagaard T, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol 2010;94:173–80.
- Van Dyk S, Narayan K, Fisher R, Bernshaw D. Conformal brachytherapy planning for cervical cancer using transabdominal ultrasound. Int J Radiat Oncol Biol Phys 2009;75:64–70.
- Mahantshetty U, Khanna N, Swamidas J, Engineer R, Thakur MH, Merchant NH, et al. Trans-abdominal ultrasound (US) and magnetic resonance imaging (MRI) correlation for conformal intracavitary brachytherapy in carcinoma of the uterine cervix. Radiother Oncol 2012;102:130–4.
- Schmid MP, Pötter R, Brader P, Kratochwil A, Goldner G, Kirchheiner K, et al. Feasibility of transrectal ultrasonography for assessment of cervical cancer. Strahlenther Onkol 2013;189:123–8.