Abstract
Aim. To investigate whether blood-based markers could be used to identify prostate cancer (PCa) patients harboring lymph node (LN) metastases. In addition, E-cadherin expression was studied within the concept of epithelial mesenchymal plasticity. Material and methods. Seventy-five patients with clinically localized PCa who underwent a superextended lymphadenectomy followed by radical prostatectomy (RP) were included in this study. Preoperative plasma/serum levels of endoglin, transforming growth factor-β1 (TGF-β1), osteopontin, vascular endothelial growth factor (VEGF), vascular cell adhesion molecule-1 (VCAM-1), and E-cadherin were measured using commercially available enzyme immunoassays in 47/75 patients and correlated with clinicopathological parameters. E-cadherin expression in the diagnostic biopsies (n = 63), RP specimens (n = 75) and LN metastases (n = 106) was examined by immunohistochemical analysis. Results. Occult LN metastases were present in almost half of the patients (37/75), with a total of 106 affected LN. Preoperative levels of endoglin, TGF-β1, osteopontin, VEGF, VCAM-1 nor E-cadherin were significantly associated with LN status. Only a positive correlation between plasma endoglin and serum prostate-specific antigen was found (Spearman's r = 0.44; p = 0.002). The majority of biopsies (91.9%) and RP specimens (79.7%) showed strong E-cadherin expression, while in the LN this was found to be much weaker (28.9%). While the staining pattern in the isolated tumor cells (ITC) and micrometastases was mainly homogenous, the macrometastases showed a much more heterogeneous pattern (χ², p < 0.0001). Conclusion. In this study, none of the blood-based markers tested could be used for nodal staging in PCa, nor could E-cadherin expression in the tissue. However, the difference in E-cadherin expression pattern between the ITC/micrometastases and the macrometastases may point to another biological behavior. The specific staining pattern seen in the macrometastases could indicate an ongoing mesenchymal epithelial transition, presumed to be a mechanism for metastatic colonization. As the latter is the rate-limiting step in the metastatic process, evaluation of the E-cadherin expression pattern could have potential therapeutic implications.
Lymph nodes on the draining lymphatic vessels from the primary tumor are often the first place of metastasis, as well as a springboard for further systemic dissemination. It is therefore evident that the presence of lymph node (LN) metastases is an important prognostic factor in many cancers and also in prostate cancer (PCa) [Citation1]. Until now, only an extended pelvic LN dissection (LND) ensures a reliable LN staging. Yet, this invasive procedure is associated with significant morbidity. Currently, sufficiently accurate non-invasive strategies to predict nodal status are lacking as the reported sensitivity levels of both conventional and functional imaging techniques are low, and even contemporary risk-stratification models have several disadvantages [Citation2,Citation3]. Much less effort has been put into the search for blood-based markers for nodal staging, although this could potentially provide an ideal strategy to identify patients at high risk for LN involvement (LNI).
Several blood-based markers have been put forward for the diagnosis, staging and prognosis of PCa [Citation4]. Many of these, including endoglin, transforming growth factor-β (TGF-β), osteopontin, vascular endothelial factor (VEGF) and vascular cell adhesion molecule-1 (VCAM-1), are related in one way or another to the process of cancer metastasis. Endoglin, also known as CD105, is a TGF-β co-receptor that is abundantly expressed on vascular endothelial cells. Shed CD105 levels may be useful as an indicator for disease progression and to identify patients at risk of recurrence and metastasis [Citation5]. TGF-β itself is an ubiquitous cytokine that plays an active role in many cellular processes and its dichotomous role in tumor progression has clearly been demonstrated. Depending on the specific context, TGF-β can promote cancer metastasis through its effects on the tumor microenvironment, enhanced invasive properties and inhibition of the cellular immune system [Citation6]. Osteopontin is a multifunctional secreted phosphoglycoprotein that has also been implicated in tumor metastasis and high levels of osteopontin in the blood are associated with poor prognosis [Citation7]. The glycoprotein VEGF promotes angiogenesis, being one of the hallmarks of cancer. Increased levels of VEGF have been reported in patients with metastatic disease [Citation8]. VCAM-1 is a transmembrane glycoprotein that is transiently expressed on the surface of endothelial cells in response to VEGF and other cytokines, and it has also been shown to function as an adhesion molecule facilitating metastasis. Moreover, serum levels of soluble VCAM-1 could serve as a surrogate clinical marker for tumor angiogenesis and disease progression [Citation9]. In general, these markers have been used to predict biochemical recurrence [Citation10], and for some of these a significant association with the presence of LN metastases has been described as well [Citation11].
Not only is the search for the ideal nodal staging technique still ongoing, also the clinical relevance of small metastatic deposits has yet to be determined. There are indications that patients with node-positive disease could be subclassified according to prognostic impact based on features such as the number of affected LN or the nodal tumor volume [Citation12]. Furthermore, more insight into the underlying biological characteristics of these metastases could help to further understand the differences in response to treatment between node-positive patients. The importance of the latter is demonstrated by the fact that therapies that have been developed for patients with overt metastases are not always efficacious in patients with micrometastatic disease and vice versa [Citation13]. A potential interesting protein within this context is E-cadherin, which is well known to be important for proper cell-cell adhesion. Decreased expression of this cell surface glycoprotein is thought to be a central event in the acquisition of an invasive and metastatic phenotype and is considered a marker of the epithelial to mesenchymal transition (EMT) process [Citation14]. Moreover, cleavage of E-cadherin has been linked to the malignant progression of PCa [Citation15] and accumulation of an 80 kDa fragment of this protein into the serum of PCa patients has been reported [Citation16].
In this study, the association of the preoperative plasma or serum levels of endoglin, TGF-β1, osteopontin, VEGF, VCAM-1, and E-cadherin with LN status was investigated in PCa patients at high risk for LNI who underwent a bilateral superextended lymphadenectomy completed by radical prostatectomy (RP). The expression of E-cadherin in the diagnostic needle biopsies, RP specimens and LN metastases was also studied by means of immunohistochemistry within the concept of epithelial mesenchymal plasticity.
Material and methods
Patients
This study is a subanalysis of a prospective imaging study designed to evaluate 11C-choline positron emission tomography (PET)-computed tomography (CT) and diffusion-weighted magnetic resonance imaging (MRI) for preoperative nodal staging in PCa patients at high risk for LNI [Citation17]. From February 2008 to February 2011, 75 consecutive patients (median age: 66 years; range 49–74 years) with localized, biopsy-proven prostate adenocarcinoma who were scheduled for retropubic RP and superextended (se)LND were enrolled. Digital rectal examination and transrectal ultrasonography (TRUS) were used to stage the primary tumor. The following inclusion criteria had to be met: 1) a risk of ≥ 10% but < 35% for LN metastases according to the Partin tables (as no predictive model existed for cT3 at the time of the study conception, cT3 tumors were considered stage cT2c); 2) no pelvic LNI on contrast-enhanced CT (i.e. ≥ 8 mm in transverse dimension); 3) no bone metastases on bone scan; 4) WHO performance status < 2; 5) no previous hormonal therapy, radiotherapy or prostatectomy; and 6) no previous/other malignancy. The study was approved by the local ethics committee and written informed consent was obtained from all patients.
Surgery and histological examination
All patients underwent a bilateral seLND, removing all LN surrounding the common iliac, external iliac and internal iliac vessels, in the obturator fossa and in the presacral region, followed by open RRP. The lateral limit consisted of the pelvic sidewall, and the medial limit was the perivesical fat. The LN specimens were collected and labeled on a standardized LN map, comprising all anatomic regions just listed. These were then, together with the RP specimen, sent to the pathology department for histological examination.
Prostatectomy specimens were entirely embedded and handled according to the guidelines [Citation18]. Gleason scores were determined and tumors were pathologically staged according to the 2002 TNM classification. In addition, tumor volume was recorded, both in absolute (ml) and relative (percentage) measures. The latter was calculated as follows: [sum of all tumor areas on the slides (cm²) * slice thickness (0.3cm) * 1.33 (shrinkage factor to correct for fixation-induced shrinking)]/prostate weight (g)*100.
LN were fixed in 6% formalin overnight. All stations were examined by palpation, visual inspection and sectioning. The lamellated (1 mm sections) LN were then embedded in paraffin and all blocks were serially sectioned until the whole LN was cut. Every 300 μ, one 5 μ section was immunohistochemically stained for pankeratin [monoclonal mouse anti- human antibody, clone AE1/AE3, Ready-to-Use, Dako, Denmark – automatical staining (Dako PT Link, Dako, Denmark)]. All slides (n = 8906) were microscopically evaluated for the presence of metastases by an experienced uropathologist (E.L.). The maximal diameter of each metastatic deposit was noted and categorized in macrometastases (macro; ≥ 2 mm), micrometastases (micro; > 0.2 mm and < 2 mm) and isolated tumor cells (ITC; ≤ 0.2 mm), in accordance with the definitions used for breast cancer.
Measurement of blood-based markers
From 47 of 75 patients, preoperative plasma and serum samples were collected at least six weeks after transrectal-guided needle biopsy, either on the day before surgery or just before the start of the surgical procedure. Blood was collected into Vacutainer® plastic K2 EDTA tubes (plasma) or Vacutainer® plastic serum tubes (serum) (Becton Dickinson, New Jersey USA). After centrifugation (10 min at 1500 × g), the supernatans, corresponding either to the plasma or serum, was aliquoted and immediately frozen and stored at −80°C in polypropylene cryopreservation vials (Sarstedt, Nümbrecht, Germany). Plasma levels of endoglin, osteopontin, soluble VCAM-1 (sVCAM-1), and VEGF were measured with the commercially available enzyme-linked immunosorbent assays (ELISA) from R&D Systems (Minneapolis, USA). Serum levels of TGF-β1 and sE-cadherin were measured with ELISA kits from respectively R&D Systems and Takara Bio Inc (Shiga, Japan). All assays were performed according to the manufacturer's protocol. All samples were run in duplicate and mean values were used for analysis.
Immunohistochemical analysis of E-cadherin
Immunohistochemical staining for E-cadherin [monoclonal mouse anti-human antibody, clone NCH-38, Ready-to-Use, Dako, Denmark – automatical staining (Dako PT Link, Dako, Denmark)] was performed on the diagnostic needle biopsies, the RP specimens and the LN.
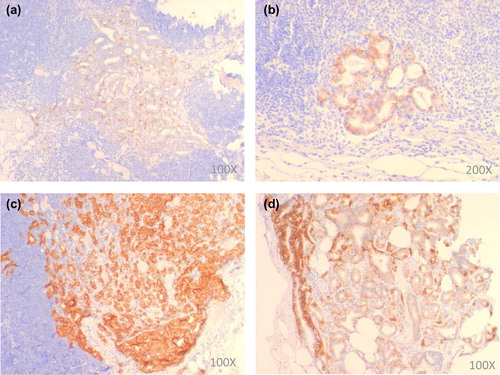
As for the diagnostic biopsies, the tissue cylinder with the highest Gleason score and the highest tumor percentage was stained for E-cadherin. For the resection specimens, the whole-mount slide containing the area determining the staging (in case of extracapsular extension/invasion of seminal vesicles) or the slide with the highest percentage of tumor (in case of pT2) was stained. All LN sections that were positive for pankeratin were also stained for E-cadherin. E-cadherin staining intensity was scored as negative/weak, moderate or strong and the staining pattern as heterogeneous (i.e. > 1 staining intensity score) or homogeneous (the same intensity score throughout the whole tumor). In case of staining heterogeneity, the most prevalent staining intensity was scored ().
Statistical analysis
All statistical analyses were performed using the software package STATISTICA 11.0® (StatSoft Inc, Tulsa, OK, USA). To compare the clinical and pathological features between node-negative and -positive patients, either the Mann-Whitney U-test or the χ²-test was performed. The Mann-Whitney U-test and Kruskal-Wallis test were used to test for differences in plasma or serum levels of the blood-based markers between clinical and pathological features. Spearman's rank correlation coefficient was used to compare ordinal and continuous variables.
To compare the E-cadherin staining intensity and staining pattern between LN negative and LN positive patients in the biopsy and prostatectomy specimens and between the different LN metastases (ITC, Micro or Macro), the χ²-test was used. To test whether the E-Cadherin staining intensity and staining pattern was linked to the size of the LN metastases, a Kruskal-Wallis test was used. Statistical significance was set as p < 0.05.
Results
Patient results and pathological analysis
summarizes patient and disease characteristics of all 75 patients enrolled in this study. At the time of inclusion, 33 (44%) patients had a diagnostic needle biopsy Gleason score ≥ 8 and 55 (73%) patients were staged ≥ cT3a. Median preoperative serum prostate-specific antigen (PSA) serum level was 10.4 ng/ml (range 1.5–70.9). Upon histological examination of the resection specimen, respectively, 32 (43%) and 42 (56%) patients had a Gleason score ≥ 8 and ≥ pT3a-stage and the median tumor volume was found to be 7.1% (range 0.4–37.0). In total, 1665 LN were removed with a median of 21 (range 7–49) nodes per patient. Step-section immunohistochemical analysis with pankeratin revealed LN metastases in almost half of the patients (37/75) and a total of 106 (6.4%) affected LN (median 2, range 1–10) was found. When taking into account only the largest metastasis per LN, 47 (44%) of these LN metastases were classified as macro (median diameter 4.0 mm, range 2.1–10.0 mm), 47 (44%) as micro (median diameter 0.673 mm, range 0.207–1.871 mm) and 12 (11%) as ITC (median diameter 0.088 mm, range 0.037–0.190 mm). When considering all metastatic deposits detected per LN, an additional 29 micros and 65 ITC were found. For all further molecular analyses, only the data on the largest metastasis per LN were used, resulting in, respectively, 23/75 (31%), 11/75 (15%) and 3/75 (4%) patients harboring macros, micros and ITC.
Table I. Patient and disease characteristics.
As expected, the presence of LN metastases correlated with pT-stage: patients with a prostate adenocarcinoma ≥ pT3a had more nodal metastases as compared to those with lower pT-stage (χ², p < 0.0001). The same held true for the Gleason score: the incidence of metastatic LN was associated with higher Gleason scores (χ², p < 0.0001). Also tumor volume was significantly higher in patients with LN metastases as compared those without (Mann-Whitney U, p < 0.05). Age, preoperative PSA levels, biopsy Gleason score and the number of LN removed per patient did not significantly differ between both patient groups, while clinical T-stage was higher in patients with positive LN (χ², p = 0.043).
Blood-based markers
From 47 of 75 patients, preoperative plasma and serum samples were available for analysis. The patient and disease characteristics from this subgroup did not significantly differ from the entire patient group. The median expression levels of the different markers tested, i.e. endoglin, osteopontin, sE-cadherin, sVCAM-1, TGF-β1 and VEGF, are summarized in . Unfortunately, for none of these, the expression level was significantly different between node-negative and -positive patients. When testing for differences in levels of these blood-based markers between clinical and pathological features, only a correlation between plasma endoglin levels and serum PSA levels was found (Spearman's r = 0.44; p = 0.002).
Table II. Preoperative plasma and serum levels of potential markers for nodal staging.
E-cadherin expression in biopsy, prostatectomy and LN specimens
An overview of all tissue samples available for analysis is depicted in Supplementary Figure 1 (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.813070). Twelve patients had their biopsy taken in another hospital, resulting in 63 patients from whom biopsies were available for E-cadherin staining. Resection specimens and LN were available from all patients. From one of the 63 diagnostic needle biopsies and one of the 75 RP specimens, the residual tissue on the E-cadherin stained section was too small for adequate evaluation. As for the LN metastases, in two cases the ITC that were present on the pankeratin stained section could not be retrieved on the E-cadherin stained section and these were therefore excluded from the analysis. The results of the immunohistochemical analysis of the E-cadherin expression in the biopsy and prostatectomy specimens on the one hand and in the different LN metastases on the other hand are shown in and , respectively.
Table III. E-cadherin staining biopsies and radical prostatectomy specimens.
Table IV. E-cadherin staining lymph node metastases.
The majority of biopsies (91.9%) and RP specimens (79.7%) showed strong E-cadherin expression. Moreover, the staining pattern was found to be mostly homogeneous, i.e. in 74.2% and 64.9%, respectively. No significant differences in the E-cadherin staining intensity or staining pattern were found between patients with negative LN and patients with positive LN ().
In general, E-cadherin expression in the LN was found to be weaker as compared to the primary tumor: negative to weak staining was observed in almost half of the LN (46.2%), while moderate to strong staining was seen in, respectively, 25.0% and 28.9% of all nodes. Also the staining pattern in the LN was much more heterogeneous as compared to the primary tumor (53.9% vs. 35.1%). While the E-cadherin staining intensity did not differ significantly between the different types of LN metastases, the staining pattern in the ITC and micros was much more homogeneous as compared to the macros (90.0% vs. 66.0% vs. 17.0%) (χ², p < 0.0001) (). For the latter, the following expression pattern was often observed: a strong expression of E-cadherin at the rim and a weaker expression in the central part of the metastasis. At last, a more heterogeneous distribution in larger lymph node metastases was found (Kruskal-Wallis, p < 0.0001) (Supplementary Figure 2, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.813070), which was in accordance with the previously mentioned different expression pattern seen in the ITC/micros versus the one in the macros.
Discussion
Despite tremendous efforts, estimating the burden of nodal disease as well as its clinical relevance still poses major challenges for the treatment of patients with PCa. The former is illustrated also in this study by the much higher frequency of LN metastases that was found, i.e. in almost half of the patients, than anticipated from the Partin tables. This could in part be explained by the fact that the Partin tables did not include patients with extracapsular extension, but even contemporary risk-stratification models may underestimate the preoperative risk for LN involvement [Citation2,Citation3]. Other strategies are therefore warranted for the upfront identification of patients harboring lymph node disease as this could aid to better stratify patients towards a more personalized and targeted approach.
Since biomarkers that can be assayed from blood will likely be easily implemented in the clinical routine, we investigated a panel of promising blood-based markers for nodal staging. More specific, we investigated the preoperative plasma or serum expression levels of endoglin, osteopontin, sE-cadherin, sVCAM-1, TGF-β1 and VEGF. This selection was based on a literature search for circulating markers thought to be important molecular determinants of PCa metastasis. Unfortunately, we were not able to demonstrate a significantly different expression level of any of these markers between those patients with LN metastases and those without. In contrast, significant associations of preoperative plasma levels of endoglin, TGF-β1 and VEGF with LN status have already been reported [Citation11,Citation19,Citation20]. Contributing factors to these conflicting results are the fact that the patient cohorts in these studies were much larger than ours and importantly, only a very limited number of patients had ≥ cT3a-stage disease as compared to our study in which about 70% of patients were staged ≥ cT3a. In a study by Kuefer and colleagues, high serum expression levels of sE-cadherin at the time of diagnosis were shown to be significantly associated with an increased risk for biochemical relapse [Citation16]. However, the authors did not assess the serum levels of this 80kDa fragment in lymph-node positive patients. In our cohort, we did not find any association of sE-cadherin levels with LN status. When testing for differences in levels of these markers between clinicopathological features, only a correlation between preoperative plasma endoglin and serum PSA was found. This is in accordance with the results of Svatek et al. who found significantly elevated preoperative plasma endoglin levels in patients with higher preoperative serum PSA levels [Citation20]. Urine-based biomarkers might represent a good alternative to blood-based markers [Citation21], but these were not investigated in this study.
In solid tumors, the dissemination of cancer cells is accompanied by the emergence of an EMT process. As stated earlier, downregulation of E-cadherin expression is considered an EMT marker [Citation14]. Concerning the expression of E-cadherin in RP specimens, conflicting data are reported in the literature, with some studies showing decreased expression of E-cadherin as tumors advance and metastasize while others did not demonstrate this relationship. In a study by Rubin et al., a high-density tissue microarray analysis of E-cadherin expression in 325 clinically localized and 97 metastatic prostate tumor samples demonstrated intermediate to intense E-cadherin expression in approximately 90% of the samples, while other studies reported aberrant E-cadherin expression in the range of 50–75% of the high grade tumors [Citation22]. Our data are in close accordance with the results of Rubin et al., as 93.2% of our RP specimens also showed moderate to strong E-cadherin expression. However, the significant association observed between aberrant E-cadherin expression and larger tumors in their study was not seen in ours.
As compared to the primary tumor, E-cadherin expression in the LN was generally found to be weaker and more heterogeneous in our study. Some other groups have found similar results [Citation23,Citation24], while others did not [Citation25]. Moreover, we observed a significant association between the E-cadherin staining pattern and the size of the metastatic deposits, with larger LN metastases showing a more heterogeneous expression pattern. This could not be merely ascribed to a volume effect, as this association was not observed for the primary tumors. The specific expression pattern that was often observed in the macros, i.e. a strong expression of E-cadherin at the rim and a weaker expression in the central part, could indicate that E-cadherin is actually re-expressed in these metastases. Altogether these data support the hypothesis of a transient downregulation of E-cadherin. At the time of invasion in the lymphatic system, EMT occurs with a concurrent loss of E-cadherin expression. However, once a critical tumor mass has been reached within the LN, it seems that a mesenchymal to epithelial reverting transition (MET) takes place. The latter is presumed to be a mechanism for metastatic colonization, which is the rate-limiting step in the metastatic process [Citation26].
Our study has several potential limitations. Foremost are the limitations inherent to any retrospective analysis. Second, our sample size is relatively small and the current follow-up is still too short to analyze the potential association of the preoperative levels of these blood-based markers with the treatment outcome. Obviously, metastasis can occur through a direct route, bypassing the LN. It is quite plausible that later correlation with biochemical disease-free survival, or indeed the occurrence of distant metastases itself, will show significant correlation with some of the tested markers, which are by no means LN specific. The same holds true for the analysis of the prognostic impact of the different types of LN metastases, i.e. ITC, micros and macros. Especially in this high-risk PCa patient cohort strong end-points such as clinical failure or time to develop castration-resistant PCa will be required in order to make any meaningful conclusions. Obviously, adjuvant therapy will have to be taken into account. Nevertheless, the very meticulous immunohistochemical analysis by means of pankeratin staining of all serially sectioned LN makes of this patient cohort a unique reference data set for pelvic LN metastasis.
In conclusion, none of the blood-based markers tested could be used for nodal staging in PCa nor could the E-cadherin expression, at least not in this patient cohort. However, the fact that a much more heterogeneous E-cadherin expression pattern was observed in the macros as compared to the ITC/micros, may point to a difference in further metastatic potential (biological behavior) and perhaps clinical relevance. The specific staining pattern seen in the macros could indicate an ongoing MET process, presumed to be a mechanism for metastatic colonization. As the latter is the rate-limiting step in the metastatic process, evaluation of the E-cadherin expression pattern could have potential therapeutic implications. For example, in case of heterogeneous E-cadherin expression in the LN metastases immediate adjuvant treatment should probably be initiated, while in case of weak and homogeneous nodal E-cadherin staining a more expectant approach could be considered. With the high incidence of PCa, the emergence of new technologies to identify lymph node metastases and the new targeted therapies to treat patients with lymph node metastasis on the rise, subclassifying patients with lymph node-positive disease will become imperative as not all patients with LN metastases seem to have an invariable poor prognosis.
Supplementary Figures 1 & 2
Download PDF (1.2 MB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported through a research grant of the ‘Stichting Emmanuel van der Schueren’ from the Flemish League against Cancer, the IWT – Institute for the Promotion of Innovation by Science and Technology in Flanders – IWT TBM 060793, and the National Cancer Plan Action 29 (KPC_ 29_037) – Belgium. K.H. is a fundamental clinical researcher of the Research Foundation – Flanders (FWO). E.L. and C.M.D. are supported by a grant from the ‘Klinisch Onderzoeksfonds (KOF)’ – University Hospitals Leuven.
References
- Gervasi LA, Mata J, Easley JD, Wilbanks JH, Seale-Hawkins C, Carlton CE Jr, et al. Prognostic significance of lymph nodal metastases in prostate cancer. J Urol 1989;142: 332–6.
- Joniau S, Van den Bergh L, Peeters C, Haustermans K, Spahn M. Nodal staging in prostate cancer: Still an unresolved issue. Eur Urol 2012;61:1139–41.
- Malmstrom PU. Lymph node staging in prostatic carcinoma revisited. Acta Oncol 2005;44:593–8.
- Shariat SF, Semjonow A, Lilja H, Savage C, Vickers AJ, Bjartell A. Tumor markers in prostate cancer I: Blood-based markers. Acta Oncol 2011;50(Suppl 1):61–75.
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J 2003;17:984–92.
- Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res 2009;19:89–102.
- Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: An effector and an effect of tumor metastasis. Curr Mol Med 2010;10:71–81.
- Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med 2011;17:347–62.
- Chen Q, Massague J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin Cancer Res 2012;18:5520–5.
- Shariat SF, Karam JA, Walz J, Roehrborn CG, Montorsi F, Margulis V, et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res 2008;14:3785–91.
- Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J Clin Oncol 2004;22:1655–63.
- Briganti A, Karnes JR, Da Pozzo LF, Cozzarini C, Gallina A, Suardi N, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol 2009;55: 261–70.
- Mina LA, Sledge GW, Jr. Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol 2011;8: 325–32.
- Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Update 2008;11:123–51.
- Giroldi LA, Schalken JA. Decreased expression of the intercellular adhesion molecule E-cadherin in prostate cancer: Biological significance and clinical implications. Cancer Metastasis Rev 1993;12:29–37.
- Kuefer R, Hofer MD, Zorn CS, Engel O, Volkmer BG, Juarez-Brito MA, et al. Assessment of a fragment of e-cadherin as a serum biomarker with predictive value for prostate cancer. Br J Cancer 2005;92:2018–23.
- Budiharto T, Joniau S, Lerut E, Van den Bergh L, Mottaghy F, Deroose CM, et al. Prospective evaluation of 11C-choline positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur Urol 2011;60:125–30.
- Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 1: Specimen handling. Mod Pathol 2011;24:6–15.
- Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, et al. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol 2001;19:2856–64.
- Svatek RS, Karam JA, Roehrborn CG, Karakiewicz PI, Slawin KM, Shariat SF. Preoperative plasma endoglin levels predict biochemical progression after radical prostatectomy. Clin Cancer Res 2008;14:3362–6.
- Roobol MJ, Haese A, Bjartell A. Tumour markers in prostate cancer III: Biomarkers in urine. Acta Oncol 2011; 50(Suppl 1):85–9.
- Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: A broad survey using high-density tissue microarray technology. Hum Pathol 2001;32:690–7.
- Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG. Expression of E-cadherin in primary and metastatic prostate cancer. Am J Pathol 1996;148: 1375–80.
- Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res 1992; 52:5104–9.
- De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology 1999;53:707–13.
- Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis 2008;25:621–8.