Abstract
Introduction. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC) is now an accepted and patient friendly treatment, but still controversy exists about its comparability to conventional radiation therapy (RT). The purpose of this single-institutional report is to describe survival outcome for medically inoperable patients with early stage NSCLC treated with SBRT compared with high dose conventional RT. Material and methods. From August 2005 to June 2012, 100 medically inoperable patients were treated with SBRT at Odense University Hospital. The thoracic RT consisted of 3 fractions (F) of 15–22 Gy delivered in nine days. For comparison a group of 32 medically inoperable patients treated with conventional RT with 80 Gy/35–40 F (5 F/week) in the period of July 1998 to August 2011 were analyzed. All tumors had histological or cytological proven NSCLC T1-2N0M0. Results. The median overall survival was 36.1 months versus 24.4 months for SBRT and conventional RT, respectively (p = 0.015). Local failure-free survival rates at one year were in SBRT group 93% versus 89% in the conventional RT group and at five years 69% versus 66%, SBRT and conventional RT respectively (p = 0.99). On multivariate analysis, female gender and performance status of 0–1 and SBRT predicted improved prognosis. Conclusion. In a cohort of patients with NSCLC there was a significant difference in overall survival favoring SBRT. Performance status of 0–1, female gender and SBRT predicted improved prognosis. However, staging procedure, confirmation procedure of recurrence and technical improvements of radiation treatment is likely to influence outcomes. However, SBRT seems to be as efficient as conventional RT and is a more convenient treatment for the patients.
Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC) is now an accepted treatment, but still controversy exists about its comparability to conventional radiation therapy (RT).
The standard treatment for patients with early stage NSCLC is surgical resection resulting in five-year local control rates between 60% and 80% [Citation1]. However, the number of elderly patients with early stage NSCLC is increasing, and in this age group comorbidity often makes surgery hazardous. The most common reasons for medical inoperability include heart disease, chronic obstructive lung disease, poor performance status and age [Citation2]. Radiotherapy is an alternative for these patients [Citation3].
Five-year local control rates for patients treated with conventional RT are typical between 30% and 50% [Citation4]. SBRT has emerged as a novel modality for lung cancer, which involves the delivery of a small number of fractions with high doses of radiation to the target volume delivered with high precision sparing the surrounding normal tissue. During the last decade, SBRT has become a more frequently used treatment for medically inoperable patients with early stage NSCLC. However, it is not known how the results can be compared with result of conventional RT.
Several studies have shown improved local control with SBRT. Lagerwaard et al. reported local control rate at one and three years of 98% and 93%, respectively, and median overall survival was 61.5 months for patients with potentially operable stage I NSCLC treated with SBRT [Citation5]. Other reports have shown similar results [Citation6–9]. With many encouraging outcomes of SBRT in retrospective studies, prospective clinical trials have been started worldwide to assess the role of SBRT in medically inoperable patients in comparison to conventional RT.
This study was made in the light of few reports describing comparison between SBRT and conventional RT. The purpose of this single-institutional report is to describe outcome for medically inoperable patients with early stage NSCLC treated with SBRT compared with high dose conventional RT.
Material and methods
Patients
From August 2005 to June 2012, 100 medically inoperable patients all with local NSCLC were treated with SBRT and 32 medically inoperable patients were treated with conventional RT with 80 Gy/35–40 fractions in the period of July 1998 to August 2011. All patients had histologically or cytologically proven NSCLC T1-2N0MO. In the conventional RT group 30 of the patients were treated up to December 2004 and the remaining two patients were treated in 2011.All patients were treated at the Department of Oncology, Odense University Hospital, Denmark. The most common reasons for medical inoperability were heart diseases, chronic obstructive lung disease or advanced age in combination with poor performance status. Data were obtained from patients’ charts and RT plans. The pretreatment evaluation for patients treated with SBRT included complete clinical examination, chest x-ray, CT scan of the chest and upper abdomen and measurement of lung function. PET/CT scan was not mandatory in staging of tumor and distant metastasis. The pretreatment evaluation for patients treated with conventional RT was the same as for SBRT-treated patients. Detailed information on staging procedure is listed in .
Table I. Patient characteristics.
Radiation therapy
Patients treated with SBRT were immobilized in a Lax-Blomgreen stereotactic body frame using a VacFix vacuum bag or similar fixation device. The patients were scanned with normal and uncoached respiration and without the use of abdominal compression. In 2007 four-dimensional (4D) CT scans were introduced to visualize the time dependence of the geometrical positions of the target volumes. The gross tumor volume (GTV) was contoured using a pulmonary CT window. Clinical target volume (CTV) is identical to GTV. Planning target volume (PTV) is defined as the CTV with a margin of 5 mm in the transversal plan and 10 mm in the longitudinal plan. The prescribed dose was 45 Gy/3 F with GTV covered by 95% (prior to October 2008) or 66 Gy/3 F in a peak in GTV. At each fraction the PTV was covered with 15 Gy. The GTV was encompassed by the 95% isodose. 32 patients were treated with the prescribed dose 45 Gy/3 F with GTV covered by 95%. One patient with 50 Gy/3 F because of tumor position in close relation to diaphragm and pleura. The treatment duration was nine days (whenever possible). Initially, the preferable treatment technique was at least six (typically 10) different coplanar beam directions with no overlapping skin entries to avoid severe skin toxicity. Since 2011 volumetric modulated arc therapy (VMAT) in two uninterrupted arcs around the patient was introduced as the preferable treatment technique. 4D cone-beam was used at each fraction to check for reproducibility of the tumor. Organs at risk (OAR) were spinal cord, esophagus, lungs, heart and nearest ribs and vertebras.
Patients treated with conventional RT received treatment five times per week. A 3D conformal radiotherapy technique was used. The Pinnacle3 system was used for treatment planning and the doses were calculated with the Collapsed-cone algorithm. Only two patients (those treated in 2011) had 4D scan performed. The GTV was contoured using a pulmonary CT window. CTV was identical to GTV. PTV was defined as a margin of 2 cm in all directions. The prescribed dose was 80 Gy in 35–40 F to cover 95% of the PTV. The treatment was without elective mediastinal nodal irradiation. Patients did not receive any chemotherapy. OAR was identical to those treated with SBRT.
Follow-up
For both groups of patients follow-up was performed five weeks after treatment, every third month in two years, and then in six-month intervals until a five-year follow-up period. The follow-up included medical history, clinical examination, chest x-ray and measurement of lung function in all patients. In case of clinical or radiological suspicion of local recurrence or metastatic disease a CT scan was performed in the SBRT group. A biopsy was not mandatory to confirm recurrence. For patients treated with conventional RT a CT scan or biopsy was not mandatory to confirm recurrence. All patients had a CT scan of chest and upper abdomen performed at each follow-up visit after January 2011.
Statistical methods
The primary endpoint of the study was overall survival. The secondary endpoints were cancer-specific survival rates and local failure-specific survival. The survival rates were calculated from the date of RT start. Overall survival was defined as the time to death from any cause including lung cancer. The cancer-specific survival was calculated by determining whether the cause of death was due to presence of pathologic or radiologic progression of NSCLC and patients who died from causes other than the NSCLC are not counted in this measurement. By review of all medical documentation at the time of death or by contacting the general practitioner, the cause of death was determined for patients not in follow-up. Local control was defined as the absence of radiologically or cytologically proven progression. The treatment outcomes were calculated using the Kaplan-Meier method. Log rank test was used for testing differences in survival rates. Multivariate analyses used Cox regression. A p-value of less than 0.05 was considered statistically significant.
The biologically equivalent doses (BED) were calculated using the following formula: BED = total dose (nd) × relative effectiveness (relative effectiveness = 1 + d/[[alpha]/[beta]]), where n is the number of fractions and d is the local dose per fraction and tumor alpha/beta ratio is 10 for lung cancer tissue [Citation10].
Results
Data was analyzed 1 March 2013. In total, 132 patients with early NSCLC were treated. The group of SBRT consists of 100 patients and the median potential follow-up time was 35.4 months (8.8–90.5 months). In the group of conventional RT, 32 patients were treated and the median potential follow-up time was 129 months (16.9–173 months). Baseline characteristics of the two patient groups are reported in . As illustrated in the median overall survival was 36.1 months versus 24.4 months for SBRT and conventional RT respectively (p = 0.02). Overall survival rate for patients treated with SBRT and conventional RT were at one year 82% versus 75%, and at five years 34% versus 10%, respectively. In a Cox multivariate analysis of overall survival female gender, PS 0–1 and SBRT were significant factors and associated with improved prognosis. While smoking status, adenocarcinoma and GTV > 25cm3 not were associated with prognosis ().
Figure 1. Kaplan-Meier survival curve of overall survival after convention radiation therapy and stereotactic body radiotherapy.
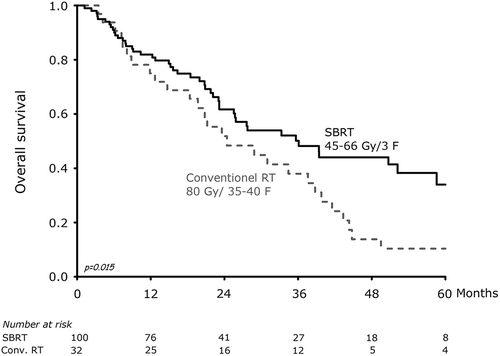
Table II. Cox multivariate regression analysis of factors affecting overall survival.
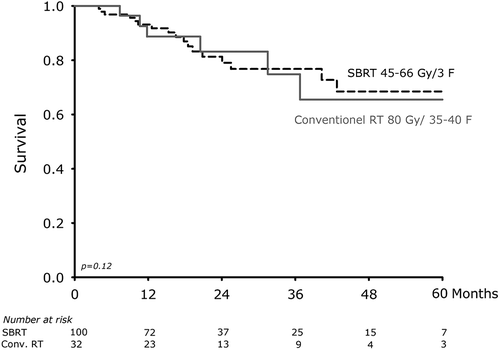
Cancer-specific survival rates for patients treated with SBRT and conventional RT were at one year 94% versus 87%, and at five years 61% versus 31%, respectively (p = 0.09). Local failure-specific survival rates at one year were in SBRT group 93% versus 89% in the conventional RT group and at five years 69% versus 66%, SBRT and conventional RT respectively (p = 0.93) (). Total local failure in the two groups occurred in 17 patients (17%) and seven patients (22%), SBRT and conventional RT, respectively (p = 0.48). Histological evaluation was performed in nine of 17 patients in the SBRT-treated patients with recurrence (four patients EBUS, three patients CT-guided biopsy, one patient lobectomy and one patient pleural effusion of malignancy). In all nine patients had confirmed recurrence by PET-scans (of which seven had made histological evaluation). CT scan of chest was performed to confirm suspicion of recurrence in two of seven patients treated with conventional RT. One patient had histological evaluation with pleural effusion of malignancy.
Figure 2. Kaplan-Meier survival curve of local-failure specific survival rates after convention radiation therapy and stereotactic body radiotherapy.
None of the patients treated in either group experienced acute toxicity except for one patient treated with conventional RT of 80 Gy/40 F who experienced self-limited severe esophagitis. There was no significant difference in decline of lung function measured by FEV1. The changes of FEV1 with 100% at start of RT in the groups were at one year and three years 97% versus 98% and 88% and 91%, SBRT and conventional RT, respectively.
Discussion
The current data set represents one of the largest single institution cohorts of patients in which all patients have biopsy proven early stage NSCLC receiving SBRT or high dose conventional RT. Most patients were considered medically inoperable in both cohorts due to heart disease or chronic obstructive lung disease with poor pulmonary function as a result.
We found that the patients treated with SBRT had a favorable survival compared with patients treated with high dose conventional RT. The median overall survival was almost one year longer for patients treated with SBRT compared to high dose conventional RT. In a previous study the difference is highest for patients in poor performance status [Citation11].
The main reason for this difference in survival is probably due to differences between the patient populations since the patients were treated in two different time periods. The staging procedures have changed. In the SBRT cohort most patients had a PET-CT scan and significantly more had an EBUS. It is therefore likely that more patients in the conventional cohort had a higher stage than was reported in this study. This view is supported by observation that tumors of the patients in the conventional cohort were larger than the tumors in SBRT cohort.
However, other factors favored the conventional treated group. There was a tendency that more patients treated with conventional RT had a good performance status. This was more or less required since the patients were about to receive radiation treatment in a schedule of 7–8 weeks. Likewise, we found a tendency of the patients in conventional group to be younger than the patients in the SBRT group, the difference being almost three years. The patients had significantly poorer lung function at the time of treatment.
No significant difference was found between SBRT and conventional RT with 80 Gy/35–40 F in terms of cancer-specific survival rates and local failure-specific survival rates in univariate analyses. In a Cox analysis of cancer specific survival including age, gender, tumor size, performance status, and smoking habit, stereotactic RT was a significant factor (data not shown). However, cancer-specific survival rates in cohorts of medically inoperable patients with comorbidities must be considered uncertain even thought the patients in this study were followed closely and all patients suspected of recurrence had a CT scan performed. However, the standard follow-up procedures for patients with no suspicion of recurrence have been changed during the time period exchanging chest x-ray with CT scans of chest and upper abdomen as the primary imaging tool in the follow-up since 2011. This change in follow-up may have resulted in an earlier recognition of local control and distant metastases.
As in previous report on SBRT, excellent local failure-specific survival rates for patients treated with SBRT for early stage NSCLC were observed [Citation6–9]. In the group of patients treated with SBRT the five-year local failure-specific survival rate was 69%. Survival in NSCLC is highly correlated with local control and that achieving local control requires doses of levels of 80–100 Gy in 2 Gy fractions [Citation12,Citation13]. The current study also shows a good local failure-specific survival rate for the group of patients treated with conventional RT. In a multi-institutional study of SBRT, it was found that a prescription of BED of 105 Gy resulted in significantly lower local recurrence rate of 4% compared with 15% for patients treated with doses above 105 Gy [Citation14]. The current study shows that the outcomes of SBRT and conventional RT were equal since five-year local failure-specific survival rates for conventional RT was 66%. A possible explanation for the high local control in the group treated with conventional RT 80 Gy/35–40 F is probably the high BED. In the current study, BED for conventional RT was 96 Gy or more. In a retrospective analysis of patients with clinical stage I NSCLC treated with definitive RT of median primary tumor dose of 63.2 Gy, five-year local failure-specific survival rate was under 15% [Citation15]. The findings in this study suggest that RT with a high BED, conventional or stereotactic, is associated with good local control. SBRT is, however, well tolerated and is more convenient for the patients due to few treatment days. Due to these findings our cohort of patients treated with SBRT of 45 Gy/3 F and 66 Gy/3 F was pooled. In a meta-analysis van Baardwijk has investigated the positive dose-response relationship for tumor control in accelerated high-dose conventional RT and SBRT and found that several schedules with equal biological doses achieve similar control rates as SBRT, and actually suggests that a reduction in dose to a level that still achieves tumor control could be possible [Citation16]. This also suggests that less toxicity of the radiation therapy will be achieved. However, in this study, only one patient experiences severe self-limited toxicity of esophagitis. The rest of patients reported no aggravation of dyspnea, coughing or esophagitis. There was not reported any significant difference in the decline lung function measured of FEV1 after treatments either. Previous reports on patients treated SBRT with peripherally located tumors and toxicity showed similar result [Citation17,Citation18]. In our cohort of patients treated with conventional RT two patients were treated after SBRT was the treatment of choice. These two patients were not suited for SBRT due to centrally located lung lesions at that time, SBRT to centrally located tumors was not performed in our institution and the standard treatment was conventional RT of 66 Gy/33 F. In 2012, our institution started treating patients with SBRT of 56 Gy/ 8 F according to a protocol of centrally located lung lesions.
In previous reports on SBRT with favorable outcomes often only patients with T1 tumors were included. In this study, patients with T2 tumors were represented in both groups of patients. The larger median gross tumor volume and higher percentage of T2 tumors in the group of conventional RT compared to the SBRT group may have had a negative impact on the difference in local control and survival rates. Soliman et al. found in a study of the impact of fractionation schedule that GTV had significant effect on locoregional control after conventional RT. An advantage with SBRT is that the compressed overall treatment time prevents accelerated repopulation of tumor cells which may add to the efficiency of the treatment [Citation19]. The patients receiving SBRT will also benefit from the minimum of treatment days and therefore obtain an almost normal daily life.
A randomized clinical trial comparing conventional RT with SBRT in patients who are unfit to undergo surgery is in progress. The Trans Tasman Radiation Oncology Group (TROG) in Australia is conducting a phase III trial (CHISEL) [Citation20] comparing 3D-CRT (60–66 Gy/30–33 F) and SBRT (54 Gy/3 F) to determine if hypofractionation is more effective, results in longer life expectancy and is just as safe as standard fractionation. Similarly, Scandinavian Stereotactic Precision And Conventional RT evaluation (SPACE) [Citation21] trial is a phase II randomized study comparing 3D-CRT (70 Gy/ 35 F) with SBRT (45 Gy/3 F). It should be noticed that SBRT dose in the SPACE trial is prescribed to the 67% of isodose level and correspond to the 66 Gy/3 F in this presented study. Until the results of these studies are mature this retrospective study on SBRT compared to high-dose conventional RT shows a potential benefit of SBRT.
Conclusion
In a cohort of patients with NSCLC there was a significant difference in overall survival favoring SBRT. There was similar outcome in secondary endpoints after SBRT and high dose conventional RT. Performance status of 0–1, female gender and SBRT predicted improved prognosis. However, staging procedure, confirmation procedure of recurrence and technical improvements of radiation treatment may have had an impact on outcomes. The main benefit of SBRT is, however, that it is a convenient treatment for the patients. Based on local control, this study suggests that the result of SBRT is at least as favorable as after conventional RT in early stage NSCLC.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60: 615–22.
- Kopek N, Paludan M, Petersen J, Hansen AT, Grau C, Høyer M. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol 2009;93:402–7.
- Janssen-Heijnen ML, Smulders S, Lemmens VE, Smeenk FW, van Geffen HJ, Coebergh JW. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax 2004;59:602–7.
- Sirzen F, Kjellen E, Sorenson S, Cavallin-Stahl E. A systematic overview of radiation therapy effects in non- small cell lung cancer. Acta Oncol 2003;42:493–515.
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, Slotman BJ, Paul MA, Smit EF, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiot Oncol Biol Phys 2012;83:348–53.
- Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung Cancer 2010;68:72–7.
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303: 1070–6.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677–82.
- Baumann P, Nyman J, Høyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6.
- Fowler JF, Tomé WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys 2004;60:1241–56.
- Jeppesen S, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus high-dose conventional radiation therapy in early-stage NSCLC: A retrospective study on local failure and survival rates. J Clin Oncol 2011;29:(Suppl; abstr 7049).
- Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324–33.
- Metha M, Manon R. Are more aggressive therapies able to improve treatment of locally advanced non-small cell lung cancer: Combined MODALITY Treatment?. Semin Oncol 2005;32:25–34.
- Grills IS, Hope AJ, Guckenberger M, Kestin LL, Werner-Wasik M, Yan D, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone- beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012;7:1382–93.
- Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1993;27:517–23.
- van Baardwijk A, Tomé WA, van Elmpt W, Bentzen SM, Reymen B, Wanders R, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill?. A systematic review. Radiother Oncol 2012;105:145–9.
- Stauder MC, Macdonald OK, Olivier KR, Call JA, Lafata K, Mayo CS, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol 2011;99:166–71.
- Baumann P, Nyman J, Hoyer M, Gagliardi G, Lax I, Wennberg B, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer – A first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol 2008;88:359–67.
- Soliman M, Yaromina A, Appold S, Zips D, Reiffenstuhl C, Schreiber A, et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: Subgroup analysis of the prospective randomized CHARTWEL trial. Radiother Oncol 2013; 106:299–344.
- Ball D. Hypofractionated radiotherapy (stereotactic) versus conventional radiotherapy for inoperable early stage I non-small cell lung cancer (NSCLC) [Internet]. The National Library of Medicine Database (US). [Updated 2009 Nov 12]. Available from http://www.clinicaltrials.gov/ct2/show/study/NCT01014130?term = CHISEL& rank = 1
- Turzer M, Brustugun OT, Waldeland E, Helland Å. Stereotactic body radiation therapy is effective and safe in patients with early-stage non-small cell lung cancer with low performance status and severe comorbidity. Case Rep Oncol 2011;4:25–34.
