Abstract
Introduction. The cure rate of early stage Hodgkin's lymphoma (HL) is excellent; investigating the late effects of treatment is thus important. Esophageal toxicity is a known side effect in patients receiving radiotherapy (RT) to the mediastinum, although little is known of this in HL survivors. This study investigates the dose to the esophagus in the treatment of early stage HL using different RT techniques. Estimated risks of early esophagitis, esophageal stricture and cancer are compared between treatments. Material and methods. We included 46 patients ≥ 15 years with supradiaphragmatic, clinical stage I–II HL, who received chemotherapy followed by involved node RT (INRT) to 30.6 Gy at our institution. INRT was planned with three-dimensional conformal RT (3DCRT). For each patient a volumetric modulated arc therapy (VMAT), proton therapy (PT) and mantle field (MF) treatment plan was simulated. Mean, maximum and minimum dose to the esophagus were extracted from the treatment plans. Risk estimates were based on dose-response models from clinical series with long-term follow-up. Statistical analyses were performed with repeated measures ANOVA using Bonferroni corrections. Results. Mean dose to the esophagus was 16.4, 16.4, 14.7 and 34.2 Gy (p < 0.001) with 3DCRT, VMAT, PT and MF treatment, respectively. No differences were seen in the estimated risk of developing esophagitis, stricture or cancer with 3DCRT compared to VMAT (p = 1.000, p = 1.000, p = 0.356). PT performed significantly better with the lowest risk estimates on all parameters compared to the photon treatments, except compared to 3DCRT for stricture (p = 0.066). On all parameters the modern techniques were superior to MF treatment (p < 0.001). Conclusions. The estimated dose to the esophagus and the corresponding estimated risks of esophageal complications are decreased significantly with highly conformal RT compared to MF treatment. The number of patients presenting with late esophageal side effects will, thus, likely be minimal in the future.
Hodgkin's lymphoma (HL) is one of the most common cancers in young adults. The median age at diagnosis is 38 years with an age-adjusted incidence rate of 2.8 per 100 000 men and women per year in the USA [Citation1]. The five-year overall survival is excellent, approaching 95% [Citation2,Citation3]. HL survivors are known to have an excess morbidity and mortality risk due to the occurrence of secondary cancers and cardiovascular diseases [Citation4–6]. However, with a growing number of young, long-term HL survivors there is a necessity of investigating other late effects of treatment.
Within the last two decades radiotherapy (RT) has changed dramatically: field size has been reduced from the extended mantle field (MF) to the involved-node RT (INRT) strategy [Citation7,Citation8], radiation dose has been reduced from 36–40 Gy to 20–30 Gy [Citation2,Citation3], and more advanced treatment techniques such as three-dimensional conformal RT (3DCRT), intensity modulated RT (IMRT), volumetric modulated arc therapy (VMAT) and proton therapy (PT) have become available. These changes have resulted in significant reductions in the radiation doses to normal structures, and this is expected to lead to a decrease in the risk of late effects. It should be noted, however, that the patients who present with late effects today, will primarily have been treated with a more extensive field (typically an MF) and will have received radiation doses up to 40 Gy.
For a large number of HL patients, part of the esophagus is within or near the border of the RT field; the esophagus is, however, rarely contoured as a dose-limiting structure, and is generally not considered an organ at risk. In other patients treated with RT to the mediastinum, early and late esophageal toxicity is a known side effect. The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) initiative reviewed 12 studies of predictors of esophageal toxicity in patients with non-small cell lung cancer (NSCLC) and found a variety of clinical and dosimetric parameters associated with early and late esophageal toxicity [Citation9]. A recent study by Morton et al. [Citation10] furthermore described the risk of treatment-related esophageal cancer among breast cancer survivors. In HL survivors, the information on late esophageal toxicity, such as the development of esophageal stricture and cancer, mainly comes from case reports [Citation11–14].
The aim of the present study is to evaluate the estimated dose to the esophagus using INRT with 3DCRT, VMAT and PT, and to compare it to the dose delivered with the extensive MF of the past for patients with early stage HL. We furthermore compare the estimated risks of developing esophagitis, esophageal stricture, and esophageal cancer with these different techniques, using dose-response models from the literature [Citation10,Citation15,Citation16].
Material and methods
Patients with clinical stage I–II HL, who received combined modality treatment with chemotherapy and INRT from 1 January 2006 to 30 August 2010 at our institution were included. Inclusion criteria were: supradiaphragmatic disease, classical histology, and age ≥ 15 years. Patients received the adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy regimen followed by INRT according to current guidelines [Citation7]. Exceptions were patients in protocol or patients unfit for this regimen. All patients had a pre-chemotherapy 18-flouro-deoxy-glucose positron emission tomography (FDG-PET) computed tomography (CT) scan as well as a post- chemotherapy CT scan for treatment planning. The two scans were fused and adjusted to the post- chemotherapy anatomical outlines. Scans were performed in the supine position with contrast enhancement and a slice thickness of 2.5 or 3.0 mm. RT doses were 30.6 Gy to initially involved lymph nodes, delivered in 1.8 Gy fractions, 5 fractions per week (F/W) with a beam energy of 6 MV. INRT was planned with 3DCRT, either as opposed or oblique fields.
Plan simulations
The simulations of VMAT and PT plans as well as the reconstructions of MF plans have been described in detail elsewhere [Citation17,Citation18]. In the planning no dose-volume constraints were applied to the esophagus (see Supplementary Table I for planning objectives, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.813636). With the modern techniques, the prescribed dose was 30.6 Gy in 1.8 Gy fractions, 5 F/W, and for MF plans the prescribed dose was 36 Gy in 1.8 Gy fractions, 5 F/W. Plans were simulated using EclipseTM v. 8.9 (Varian Medical Systems, Palo Alto, CA, USA).
Table I. Patient characteristics.
Contouring
The esophagus was defined as beginning at the lower edge of the cricoid cartilage and ending at the gastro-esophageal junction. The external contour was delineated in its entire length, by the same physician on each CT image for all patients. All contours were reviewed by a second physician as there are currently no specific recommendations for contouring the esophagus [Citation9].
Risk estimates
Risk estimates were based on dose-response models from the literature. The risk of developing esophagitis (grade ≥ 2) was based on a Lyman model derived by Belderbos et al. [Citation15] from two pooled, prospective studies of NSCLC patients. The risk of developing esophageal stricture was based on a relative seriality model by Alevronta et al. [Citation16] from a case-control study with head and neck cancer patients. The risk of developing esophageal cancer was derived from the Morton study [Citation10] using a linear dose-response model. Risk estimates were based on each patient's four different treatment plans.
Statistical analyses
Mean, maximum and minimum dose to the esophagus and estimated risks of developing esophagitis, esophageal stricture and esophageal cancer with 3DCRT, VMAT, PT and MF treatment were compared using repeated measures analysis of variance (rANOVA). When sphericity was violated the Greenhouse-Geisser correction was used. To compare the results among the independent variables post hoc analyses using the Bonferroni correction were performed. All statistical analyses were performed using SPSS Statistics v. 20 (IBM).
Results
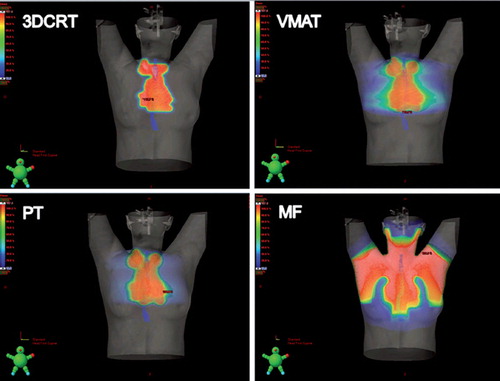
A total of 46 patients are included in the study; characteristics are shown in . shows the constructed 3DCRT, VMAT, PT and MF treatment plans for one representative patient.
Figure 1. Treatment plans with 3DCRT, VMAT, PT and MF for one patient shown in dose color wash. The esophagus is delinated in blue.
Dose distribution to the esophagus
The distribution of mean, maximum and minimum dose to the esophagus for each patient with the four different treatment techniques are shown in . Five patients receive a maximum dose to the esophagus below 1.8 Gy in their initial 3DCRT treatment plan and they are seen as outliers in . Results are shown in without outliers. All doses are reported as median values. The range in mean dose to the individual patients is wide for all but the MF plans, where the dose is more evenly distributed. Post hoc tests reveal that the difference in mean dose between 3DCRT and VMAT is not statistically significant. Pair-wise comparisons between 3DCRT versus PT and VMAT versus PT do, however, translate into a significant difference. The mean dose delivered to the esophagus is significantly higher for MF compared with the three modern techniques. Regarding maximum dose to the esophagus, wide differences in range are detected for all but the MF plans. No statistically significant differences are seen when the three modern techniques are compared. Maximum dose to the esophagus is significantly higher with the extensive MF treatment when compared to the modern techniques. For the minimum dose to the esophagus, the pair-wise comparisons between all treatment plans translate into a statistically significant difference in favor of the modern techniques.
Figure 2. Minimum, mean and maximum dose distribution to the esophagus with 3DCRT, VMAT, PT and MF treatment. Boxes represent the 25th to 75th percentiles; whiskers represent the range, the plus signs represent outliers.
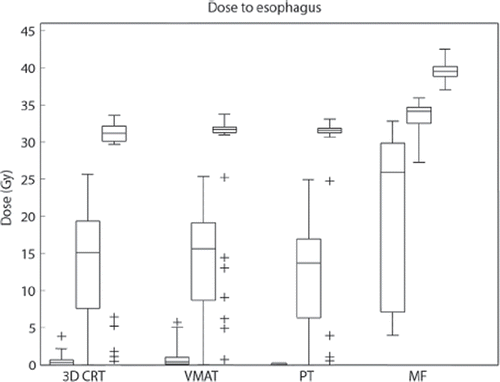
Table II. Mean, maximum and minimum dose to the esophagus with 3DCRT, VMAT, PT and MF treatment plans without outliers and p-values for all comparisons and their pair-wise comparisons. Doses are reported as median values for the whole group.
Risk estimates
The risk estimates are shown in . The modern techniques provide significantly lower risk estimates compared with the extensive MF treatment on all parameters. The patients’ estimated risks of developing esophagitis decrease approximately five-fold with the modern techniques, esophageal stricture 500-fold, and esophageal cancer seven-fold compared to the MF treatment. Comparison of 3DCRT versus VMAT is not significantly different for esophagitis, esophageal stricture or cancer in post hoc tests. The same is true for esophageal stricture when 3DCRT is compared with PT, but not when VMAT is compared with PT. All risk estimates are minimal with the modern techniques and only the early endpoint esophagitis carries a median risk above 1%.
Table III. Risk estimates with 3DCRT, VMAT, PT and MF treatment plans and p-values for all comparisons and their pair-wise comparisons.
Discussion
The focus of this study is to investigate the radiation dose to the esophagus in early stage HL, using different RT techniques. Dose-response models are applied to estimate the risk of developing esophagitis and late effects in the form of esophageal stricture and esophageal cancer. Comparisons are made between INRT, delivered as 3DCRT, VMAT, or PT and the extensive MF technique. By comparing the modern techniques with the outdated MF technique, it can be estimated whether today's patients share the same risk of late effects.
Esophagitis
Early esophagitis (occurring < 90 days after treatment initiation) is a known side effect in patients undergoing RT for thoracic tumors [Citation9,Citation15,Citation19]. Results from the QUANTEC study [Citation9] show a clear trend demonstrating that volumes receiving > 40–50 Gy correlate significantly with early esophagitis. Factors increasing the risk of esophagitis are increased RT aggressiveness (e.g. hyperfractionation, concurrent boost), concurrent chemotherapy and clinical risk factors, such as pre-existing dysphagia and increasing nodal stage. In the present study, the mean dose to the esophagus is 34.2 Gy with MF and ≤ 16.4 Gy with the three modern techniques. Corresponding maximum dose to the esophagus is 39.6 Gy with MF and ≤ 31.8 Gy with the modern techniques. This is expected to reduce the risk of esophagitis from 22.6% with MF to approximately 4% with the modern techniques. One patient is however estimated to receive a mean esophageal dose of approximately 25 Gy and a maximum dose of approximately 32 Gy, regardless of which of the modern techniques are used, resulting in an estimated risk of esophagitis of approximately 11%. The Belderbos study [Citation15] finds that when accepting a 30% risk of developing esophagitis grade ≥ 2, a maximum volume of 50% of the esophagus can be irradiated to 35 Gy. This dose/volume recommendation for the esophagus is also given by QUANTEC [Citation20]. Our results indicate that, in the treatment of HL, highly conformal RT reduces the risk of esophagitis to a minor problem.
Esophageal stricture
For HL patients, the literature on late esophageal damage with fibrosis leading to stricture, stenosis and associated dysphagia, trachea-esophageal fistulas, and/or perforation is sparse. Esophageal stricture has been reported in pediatric patients with HL 10–15 years after MF treatment with a radiation dose ≥ 40 Gy, however, in one case the patient received only 20 Gy [Citation11,Citation12]. In adults with head and neck cancer and NSCLC, esophageal stricture is a known complication [Citation16,Citation21,Citation22]. Ahn et al. [Citation22] find that the only statistically significant clinical parameter for developing any grade of late esophageal toxicity is the severity of the acute esophageal toxicity, suggesting that this early endpoint might be causally correlated (i.e. a consequential late effect). This raises the question if more aggressive supportive care to patients can help prevent this severe late event. It would be encouraging to see a clinical outcome series demonstrating a drop in early esophagitis with modern techniques, as this would be expected to be followed by a later drop in the risk of esophageal stricture. In a study by Lawson et al. [Citation21], esophageal stricture developed in 13% of the patients after 52% of the esophagus received a mean dose of 60 Gy. In the group without stricture, only 30% of the esophagus was irradiated to 60 Gy. The dose used in modern day treatment of HL is well below 40 Gy [Citation2,Citation3], and the estimated risk of < 0.01% of developing esophageal stricture with the modern techniques in our study reflects this.
Esophageal cancer
The criteria for radiation-induced malignancies of the esophagus were described in 1972 by Chudecki [Citation23]. Micke et al. [Citation14] reviewed the literature and found 66 case reports of radiation-induced esophageal cancer from 1957 to 1994. Patients with previous breast cancer (17 patients) and HL (12 patients) accounted for the majority of these reports. Esophageal cancer in a previously irradiated area occurred with a median time-to-event of 15 years and median dose of 40 Gy. They were not able to confirm the diagnosis of radiation-induced esophageal cancer versus spontaneously developed cancer.
Analyses supported a time-dose relationship in which higher doses shortened the latency interval; however, this effect was not significant. Morton et al. [Citation10] find that, in breast cancer survivors who received radiation to the site of the esophageal tumor, the risk of esophageal cancer increases 8.3-fold for doses ≥ 35 Gy. The median time-to-event between breast cancer and esophageal cancer is 13 years (range 5–37 years), and the risk increases with known esophageal cancer risk factors such as smoking, alcohol consumption, family history of cancer and increasing BMI. The overall risk is, however, low: 0.5% risk of developing esophageal cancer within 25 years after irradiation to 30 Gy.
In the present study, the estimated maximum dose to the esophagus is ≤ 31.8 Gy with a corresponding estimated risk of developing esophageal cancer < 0.2% with the three modern techniques. With the MF technique, mean and maximum dose are 34.2 Gy and 39.6 Gy, respectively, increasing the risk of developing esophageal cancer approximately seven-fold. Morton [Citation10] recommends that patients who receive irradiation to the mediastinum should refrain from smoking and be thoroughly examined with gastroscopy if presenting with dysphagia. This recommendation could be transferred to HL survivors, to enable early detection of esophageal cancer.
Choosing the optimal treatment technique
A study by Gomez et al. [Citation19] shows that patients with NSCLC treated with IMRT has the highest rate of grade 3 acute esophagitis when compared with 3DCRT and PT. The authors speculate that this could be due to the “low-dose bath” received by the esophagus with IMRT, whereas 3DCRT and PT allow complete or partial sparing of the esophagus. Fiandra et al. [Citation24] find IMRT techniques to be superior in target coverage and organ at risk-sparing compared to 3DCRT, with, as expected, larger volumes of healthy tissues receiving low-intermediate doses. Considering all the different organs at risk simultaneously, they are not able to find an optimal IMRT technique.
In the present study we find no statistically significant differences in the estimated mean dose to the esophagus or in the risk estimates of developing esophagitis, esophageal stricture, or esophageal cancer when 3DCR is compared to VMAT. The “low-dose bath” delivered with VMAT can, however, potentially increase the risk of morbidity and secondary cancer in surrounding organs, and this question needs further investigation. PT performs significantly better with the lowest risk estimates on all parameters compared to the photon treatments, except when compared to 3DCRT for stricture. Some patients will however receive a high radiation dose to the esophagus regardless of the modern technique used, and the optimal choice of treatment technique must therefore be assessed for each individual patient. However, the absolute risk estimates are so low, that in most patients the radiation dose to the esophagus is unlikely to be the most important endpoint when choosing treatment strategy. Other risk organs in the mediastinum, such as the heart, lungs, or breasts for female patients are of more concern than the esophagus. However, as recently shown by our group, when considering these organs we found no one single best modern RT technique due to the anatomically diversity among HL patients [Citation18].
Our study has the inherent limitation of a dose planning study: The esophagus is slightly mobile during respiration which is unaccounted for, and there are uncertainties in the delineation of the esophagus. Also, no dose-volume constraints are applied to the esophagus in the treatment planning. A further limitation is that the risk estimates are based on data from other patient groups, whose treatment differ from that of HL patients, making the direct transfer of the risk estimates to our patient cohort questionable. However, all treatment plans share these limitations and they should not systematically affect our conclusions. Also, the direct comparison between MF and the modern INRT techniques is biased due to the difference in field size and dose. However, this reflects the change in treatment regimen, and the comparison is important in order to extrapolate the risk from earlier time periods to patients of today.
Conclusion
The results of this study show that modern INRT treatment for early stage HL with 3DCRT, VMAT or PT significantly reduces the estimated dose to the esophagus and the corresponding estimated risks of esophageal complications when compared to the extensive MF treatment. This will likely decrease the number of HL survivors presenting with esophageal side effects in the future. Possibly, the risk of esophageal late effects can be further reduced with cessation of smoking and excessive alcohol intake, and with thorough clinical examination of HL survivors presenting with dysphagia, to ensure early detection.
Supplementary Table I
Download PDF (594.8 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- National Cancer Institute. SEER State Fact Sheets: Hodgkin Lymphoma. SEER Cancer Statistics Review November 2011. [Internet]. Available from: http://seer.cancer.gov/statfacts/html/hodg.html. [cited 2013 Jan 14].
- Eich HT, Diehl V, Gorgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010; 28:4199–206.
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med 2010; 363:640–52.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003;21:3431–9.
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–86.
- Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol 2002;20:2101–8.
- Girinsky T, van der Maazen R, Specht L, Aleman B, Poortmans P, Lievens Y, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: Concepts and guidelines. Radiother Oncol 2006;79:270–7.
- Maraldo MV, Aznar MC, Vogelius IR, Petersen PM, Specht L. Involved node radiation therapy: An effective alternative in early-stage Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2013;85:1057–65.
- Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S86–93.
- Morton LM, Gilbert ES, Hall P, Andersson M, Joensuu H, Vaalavirta L, et al. Risk of treatment-related esophageal cancer among breast cancer survivors. Ann Oncol 2012; 23:3081–91.
- Ellenhorn JD, Lambroza A, Lindsley KL, LaQuaglia MP. Treatment-related sophageal stricture in pediatric patients with cancer. Cancer 1993;71:4084–90.
- Mahboubi S, Silber JH. Radiation-induced esophageal strictures in children with cancer. Eur Radiol 1997;7:119–22.
- Schiavetti A, Di NG, Ingrosso A, Chiriaco D, Cucchiara S. Barrett esophagus in long-term survivors of childhood solid tumors. J Pediatr Hematol Oncol 2011;33:559–61.
- Micke O, Schafer U, Glashorster M, Prott FJ, Willich N. Radiation-induced esophageal carcinoma 30 years after mediastinal irradiation: Case report and review of the literature. Jpn J Clin Oncol 1999;29:164–70.
- Belderbos J, Heemsbergen W, Hoogeman M, Pengel K, Rossi M, Lebesque J. Acute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol 2005;75:157–64.
- Alevronta E, Ahlberg A, Mavroidis P, al-Abany M, Friesland S, Tilikidis A, et al. Dose-response relations for stricture in the proximal oesophagus from head and neck radiotherapy. Radiother Oncol 2010;97:54–9.
- Maraldo MV, Brodin NP, Vogelius IR, Aznar MC, Munck af RP, Petersen PM, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012; 83:1232–7.
- Maraldo MV, Brodin NP, Aznar MC, Vogelius IR, Munck af RP, Petersen PM, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol Epub 2013 Apr 25.
- Gomez DR, Tucker SL, Martel MK, Mohan R, Balter PA, Lopez Guerra JL, et al. Predictors of high-grade esophagitis after definitive three-dimensional conformal therapy, intensity-modulated radiation therapy, or proton beam therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;84:1010–6.
- Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S10–9.
- Lawson JD, Otto K, Grist W, Johnstone PA. Frequency of esophageal stenosis after simultaneous modulated accelerated radiation therapy and chemotherapy for head and neck cancer. Am J Otolaryngol 2008;29:13–9.
- Ahn SJ, Kahn D, Zhou S, Yu X, Hollis D, Shafman TD, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys 2005;61:335–47.
- Chudecki B. Radiation cancer of the thoracic oesophagus. Br J Radiol 1972;45:303–4.
- Fiandra C, Filippi AR, Catuzzo P, Botticella A, Ciammella P, Franco P, et al. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s lymphoma: Dosimetric comparison and clinical considerations. Radiat Oncol 2012;7:186.
