Abstract
Background. Adaptive radiation therapy (ART) for urinary bladder cancer has emerged as a promising alternative to conventional RT with potential to minimize radiation-induced toxicity to healthy tissues. In this work we have studied bladder volume variations and their effect on healthy bladder dose sparing and intrafractional margins, in order to refine our ART strategy. Material and methods. An online ART treatment strategy was followed for five patients with urinary bladder cancer with the tumors demarcated using Lipiodol®. A library of 3–4 predefined treatment plans for each patient was created based on four successive computed tomography (CT) scans. Cone beam CT (CBCT) images were acquired before each treatment fraction and after the treatment at least weekly. In partial bladder treatment the sparing of the healthy part of the bladder was investigated. The bladder wall displacements due to bladder filling were determined in three orthogonal directions (CC, AP, DEX-SIN) using the treatment planning CT scans. An ellipsoidal model was applied in order to find the theoretical maximum values for the bladder wall displacements. Moreover, the actual bladder filling rate during treatment was evaluated using the CBCT images. Results. In partial bladder treatment the volume of the bladder receiving high absorbed doses was generally smaller with a full than empty bladder. The estimation of the bladder volume and the upper limit for the intrafractional movement of the bladder wall could be represented with an ellipsoidal model with a reasonable accuracy. Observed maximum growth of bladder dimensions was less than 10 mm in all three orthogonal directions during 15 minute interval. Conclusion. The use of Lipiodol contrast agent enables partial bladder treatment with reduced irradiation of the healthy bladder volume. The ellipsoidal bladder model can be used for the estimation of the bladder volume changes and the upper limit of the bladder wall movement during the treatment fraction.
Radiation therapy (RT) for urinary bladder cancer has rapidly developed during recent years due to the implementation of cone beam computed tomography (CBCT) into the clinical routine. In the conventional treatment approach without CBCT imaging large margins are added around the bladder to create the planning target volume (PTV), in order to account for the variations of bladder volume and position during the treatment [Citation1,Citation2]. This may lead to increased gastro-intestinal toxicity, as large volumes of normal tissues, such as small bowel, receive high radiation doses [Citation3]. Target visualization prior to each treatment fraction has recently improved due to increased use of CBCT. Consequently, smaller PTV margins have been derived and reported [Citation4,Citation5], and the tracking of individual bladder filling patterns has led to development of adaptive radiation therapy (ART) techniques for bladder cancer.
The tumor delineation in the treatment planning CT can be problematic in bladder carcinoma due to the possible preceding procedures such as trans- urethral resection of the tumor or neoadjuvant chemotherapy. One method of enhancing the visibility of the tumor site in bladder wall is the use of Lipiodol®, poppyseed oil that acts as a contrast agent. Small drops of Lipiodol can be injected into the bladder wall during cystoscopy to mark the borders of the tumor. These Lipiodol markers can then be used to aid the contouring of the gross tumor volume (GTV) and in the process of CBCT image registration [Citation6]. Normally, without contrast agent the tumor cannot be reliably detected in CBCT images due to relatively poor image quality. The injection of contrast agent around the tumor enhances its visibility and thus enables the treatment of partial bladder [Citation6,Citation7].
Different approaches of dealing with the daily changes in bladder volume have been introduced in literature. ART for bladder cancer has been reported using both offline and online strategies for treatment plan adaptation [Citation8–13]. The online adaptive approach may include several planning CT scans and creation of a library of treatment plans [Citation10–12]. Some groups have created adaptive planning target volumes (PTV) based on CBCT images acquired during the first fractions of the treatment [Citation9,Citation13]. In this approach the first week of a treatment course is performed with a non-adaptive treatment delivery.
Our group was among the first to report clinical implementation of online ART for bladder cancer. In our previous work [Citation10] a substantial reduction in dose to the small bowel was achieved using a predefined selection of multiple intensity-modulated radiation therapy (IMRT) treatment plans when compared to non-adaptive treatment delivery with a single IMRT plan. In addition, dose coverage in the treatment of the whole bladder was shown to be similar in ART as in the non-adaptive treatment. In this study further optimization of our clinical treatment strategy is carried out. The aim of the research was to evaluate intrafractional bladder filling by observing the changes of bladder dimensions between successive treatment planning CT scans. Another goal was to report recent improvements in our clinical treatment protocol, including partial bladder treatment enabled by Lipiodol and use of low dose CBCT protocols.
Material and methods
CT imaging and treatment planning
In this study five patients with muscle invasive T2-T4a bladder cancer considered unfit for cystectomy were treated using online ART. The plan of the day was selected from a library of volumetric-modulated arc therapy (VMAT) treatment plans. All patients had Lipiodol markers injected to the bladder wall during cystoscopy by the urologist 10–30 days before RT. For the treatment planning a sequence of four repeated CT scans (GE LightSpeed RT, GE Medical Systems Inc., Waukesha, WI, USA) was acquired with an interval of 15 minutes between the scans. Prior to the first scan the patients emptied their bladder and drank 8 dl of water. The planning CT images were co-registered using bone anatomy. For all the four CTs with different bladder volumes a complete set of contours was generated including CTVs, PTVs, bladder (if not equal to CTV), rectum, intestinal cavity and femoral heads. These structures were copied into the first planning CT with an empty bladder, which was used as the basis for treatment plan calculations. A library of 3–4 treatment plans was generated using the different PTVs and OARs. If bladder expansion during the sequence of planning CTs was modest, one of the middle-sized PTVs was left unused.
Three patients received a partial bladder treatment with the CTV comprising the Lipiodol-marked area plus 1 cm of the surrounding bladder wall, expanded isotropically by 10 mm to create the PTV. For two patients the treatment was divided in two parts: the elective treatment with the CTV including the whole bladder and areas of extravesical extension, and the boost treatment with the CTV similar to the partial bladder treatment. Anisotropic margins of 10 mm in anterior and cranial direction and 15 mm in lateral, posterior and caudal direction were used to create the whole bladder PTVs. Prescription doses varied according to the treatment volume. In partial bladder treatment a fractionation of 52.5 Gy/2.5 Gy was used. In elective treatment to the whole bladder 44 Gy was given using 2 Gy fraction size and the boost volume received 20 Gy using 2 Gy dose per fraction.
Treatment planning was performed in the Monaco treatment planning system (Elekta AB, Stockholm, Sweden), using VMAT with 6 MV or 10 MV photon energies for both whole and partial bladder PTVs. For the dosimetric analysis of the partial bladder treatment the DVHs for bladder were compared for the different treatment plans with varying bladder filling.
Image guidance and plan selection
The patients were treated with the Elekta Axesse linear accelerator and the CBCT images were acquired using the XVI imaging system (Elekta AB, Stockholm, Sweden). Before the beginning of the treatment fraction the patients emptied their bladder, and no specific drinking instructions were given. A CBCT image was acquired prior to each fraction and registered with the treatment planning CT image of the empty bladder with the bladder, CTV and PTV contours copied from all four planning CTs. Automatic registration was performed using gray value information in a region of interest including the bladder and its immediate surroundings, and the results were visually inspected by radiation oncologist or physicist. The bladder volume before the fraction was compared to the different sized bladder contours in the planning CT, and the appropriate treatment plan was chosen by selecting the plan where bladder was fully encompassed by the smallest possible bladder contour. Additionally, in partial bladder treatment it was made sure that the area outlined by Lipiodol was encompassed by the CTV associated with the selected treatment plan. For all five patients a CBCT image was also acquired after the treatment fraction for the purpose of imaging dose optimization and assessment of intrafractional filling of the bladder. The post-treatment CBCTs were acquired during the first treatment fractions and subsequently at least weekly.
We developed a method to minimize the CBCT imaging dose without compromising the visibility of the bladder wall and while maintaining the automatic matching accuracy of XVI software [Citation14]. Three low dose CBCT protocols were generated, tested in a phantom study [Citation14] and implemented in the bladder ART by the following method: The first CBCT image prior to the first treatment fraction was taken using manufacturer presets for imaging parameters. The next CBCT image was acquired after the first treatment fraction, using adjusted imaging parameters (such as kV, mAs) to achieve lower dose. If the image quality was sufficient with the low dose protocol to choose the daily treatment plan for the particular patient, this imaging protocol was then used in the next treatment fraction. Following this procedure the CBCT imaging dose could be lowered at each fraction by applying higher dose imaging protocol before and lower dose imaging protocol after treatment. This was continued until the protocol with the lowest imaging dose was reached or as long as the image quality was seen to be adequate.
Intrafractional change of the bladder volume and dimensions
To estimate appropriate margins for intrafractional changes of bladder size a simple ellipsoidal model was used where the maximal dimensions of bladder in cranial-caudal (z), anterior-posterior (y) and lateral (x) directions were measured from the planning CT scans. The measurements were performed relative to the anatomical “base” in order to observe possible asymmetrical changes in the bladder dimensions. In cranial-caudal direction this was the most caudal point in the empty bladder wall, and in anterior-posterior direction the most posterior point in the empty bladder wall. The origin for the measurements in lateral direction was the symphysis midline. In this model the bladder volume Ve was calculated as
where the dimensions z, y and x are measured relative to the anatomical origin. The ellipsoidal volume Ve was compared with the actual bladder volume VCT defined from the planning CT scans.
In order to find an upper limit for possible bladder wall displacements during a typical treatment fraction of 15 minutes, an assumption was made that the bladder grows only in one dimension while the other two orthogonal dimensions are kept constant. Instead of giving an accurate description of the bladder growth dynamics, this approach gives a worst case scenario for bladder wall displacements. Nevertheless, from the previous one dimensional growth assumption it is possible to calculate a theoretical limit for the maximum change ∆z (or x,y) in that direction from the volume change ∆V by partially differentiating Equation 1 with respect to z while x and y are constant
which shows that as the volume (V ∝ xyz) is increasing the rate of the change of bladder size is decreasing. If the bladder expansion is assumed isotropic in all directions (Δz ≈ Δy ≈ Δx), the wall displacement is 1/3 of the maximum change given by Equation 2,
The elliptical bladder model and the worst case scenario of intrafractional change of the bladder size were compared with the actual bladder volume and changes in bladder wall extreme positions in the planning CT scans of five patients.
The actual intrafraction filling during the treatment was measured from CBCT images. Five pre- and post-treatment CBCT images taken approximately one week apart were analyzed for each patient. The bladder contours were delineated in the CBCT images, and the filling rate for the bladder was calculated between the pre- and post-fraction CBCT images. This was achieved by dividing the difference in bladder volumes between pre- and post-fraction images by the time elapsed between the two acquisitions. For comparison the bladder filling rate during the treatment planning CT sequence was determined as the average of all successive scans.
Results
Intrafractional bladder filling
In the bladder volume is modeled with the ellipsoidal shape as a function of true bladder volume determined from the planning CT scans from five patients. The ellipsoidal model estimates the true volume with a relatively good accuracy. The observed dimensional changes are in general less than the change calculated from the worst case scenario where the volume growth is modeled as a mere consequence of growth in one dimension (1D; Equation 2), and all observed values are less than 10 mm ().
Figure 1. A) Comparison of the bladder volume modeled as an ellipsoid and the true bladder volume in planning CT scans. B) Observed changes of the maximum dimensions of the bladder in cranial-caudal (C-C), anterior-posterior (A-P) and lateral (DX-SIN) directions (open symbols). All observed changes lie under the level of 1.0 cm (dotted line). The theoretical limit for the growth in one dimension (one of C-C, A-P or DX-SIN, others unchanged) is plotted in solid spheres (fit: solid line) and the theoretical growth in three dimensions (all of C-C, A-P and DX-SIN) is plotted with solid squares (fit: dashed line) (Equations 2 and 3).
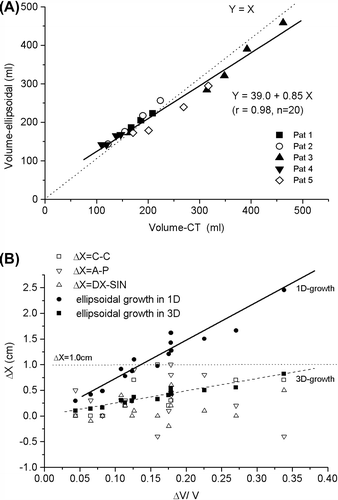
The observed changes in bladder diameter between successive CT scans in 15 minute intervals were 0.38 ± 0.29 cm (mean ± 1 SD), maximum 1.0 cm in cranial-caudal direction, 0.34 ± 0.42 cm, maximum 0.9 cm, in anterior-posterior direction and 0.15 ± 0.24 cm, maximum 0.5 cm in lateral direction, respectively. The growth was quite symmetric in anterior-posterior and lateral dimensions, and therefore, CTV-ITV (internal target volume) margin can be divided as a half of the diameter increase in both sides. However, the caudal wall of the bladder did not practically move at all while the cranial part showed the majority of the movement in cranial caudal direction (ITV margin added only in the cranial border to cover the whole diameter increase). In all the observed bladder dimensions expanded less than 10 mm within a 15 minute interval. If the online positioning correction before treatment is performed by centering the bladder visible in CBCT in the middle of the selected bladder contour, the 10 mm margin can be divided in half (5 mm) to give the intrafractional margin in lateral and anterior-posterior directions, as was stated earlier. In our treatment setup a 10 mm margin should be added in cranial direction to account for intrafractional filling and bladder deformation, and the margin in caudal direction can be less than 0.3 cm. These margins are only adequate for a maximum time of 15 minutes elapsed from acquisition of CBCT to the end of irradiation.
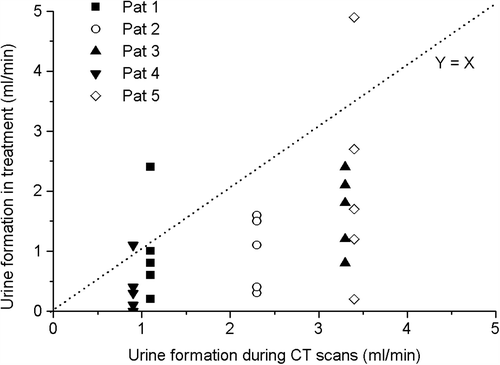
In addition to the analysis of bladder wall displacements, we also followed the rate of the urine formation. From the bladder volumetric changes between successive planning CT scans we calculated the rate of the bladder volume increase to vary between 0.46 and 4.6 ml/min (mean± SD = 2.1 ± 1.3 ml/min) despite the aimed controlled conditions for hydration. There was also a considerable variation in urine formation during the entire 45 minute planning CT scanning procedure of an individual patient (four successive CTs).
In , the intrafractional bladder filling rates during treatment, determined using CBCT images, are presented as a function of average bladder filling rate as seen in treatment planning CT images. This image shows that the excretion of urine into the bladder was mostly lower during the treatment than during treatment planning CT. On two of 25 occasions the observed bladder filling exceeded clearly the expected values.
Partial bladder treatment
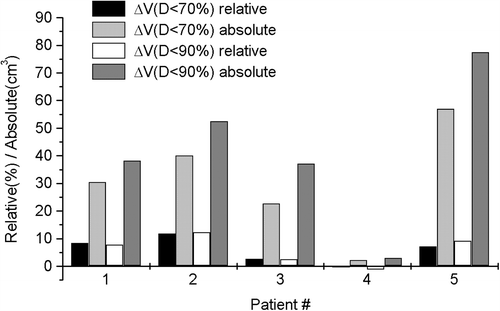
The effect of the bladder volume (empty/full) on the sparing of the bladder in partial bladder treatment is demonstrated in . For three patients it is obvious that the optimal treatment is given with a full bladder, regarding only the dose received by the bladder. One patient had only small difference in bladder DVHs between the full and empty bladder. In addition, for one patient it seems that treatment with an empty bladder would give lower dose to the bladder. This can be explained by particularly slow bladder filling during treatment planning CT, thus creating only minor differences between the bladder volumes.
CBCT dose optimization
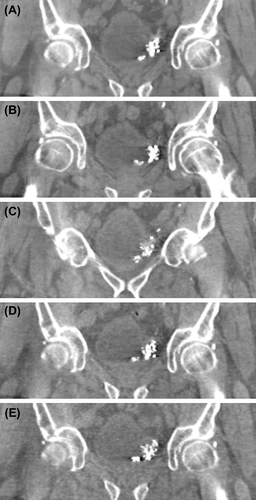
For two patients the dose minimizing scheme was followed to the imaging protocol with the lowest dose. By using an optimized imaging protocol instead of manufacturer protocols, the absorbed dose measured in the center of a phantom (PMMA, cylindrical, r = 16 cm) could be reduced by 85%, from 20.5 mGy to as low as 3 mGy per fraction (). For two other patients the imaging dose was reduced by 63%. One patient was severely obese and the default CBCT protocol with the highest imaging dose was utilized in order to achieve good image quality.
Figure 4. Five coronal views of CBCT images from the same patient using different imaging protocols. The absorbed doses measured in the center of a PMMA phantom (r = 16cm) for the protocols are A) 20.5 mGy B) 11.2 mGy C) 7.5 mGy D) 4.0 mGy and E) 3.0 mGy. A and B are manufacturer protocols and C, D and E are optimized low dose protocols. For this patient the image quality with protocol E was decided sufficient for treatment plan selection.
Discussion
Adaptive treatment protocol for bladder cancer has previously been shown to reduce dose to small bowel while maintaining sufficient dose coverage in PTV [Citation5,Citation10]. In our clinic we have adopted the strategy of repetitive CT scanning and creation of multiple treatment plans for the patients. Alternative approach reported by Meijer et al. [Citation11] is to take treatment planning scans with full and empty bladder and interpolating medium size PTVs between the extremes and also extrapolating the largest PTV. Another strategy is to compose the adaptive PTVs using the CBCT images from the first week of treatment given with a non-adaptive technique, as has been suggested by Vestergaard et al. and Foroudi et al. [Citation9,Citation13]. It would be useful to compare these techniques with our approach.
The optimal filling in the treatment of partial bladder was investigated in order to be able to spare a part of the bladder. Three patients of five would have benefited from treatment with a full bladder. For two patients the difference between irradiated volumes of bladder in treatment with full and empty bladder was negligible. For one of these two patients the bladder filling rate in treatment planning CT was very low. As there are no proper recommendations for the bladder dose volume tolerances under 60 Gy dose level given in the literature, it is difficult to conclude whether the differences in the sparing of the bladder with varying bladder volumes is clinically relevant. Although full bladder seems optimal for partial bladder treatment regarding the dose sparing in the healthy part of the bladder, dose to other OARs (such as rectum and small bowel), which were not evaluated in our research should also be taken into account. Moreover, in this study the ART and non-adaptive RT techniques were not compared with respect to normal tissue irradiation, but it is clear that larger CTV-PTV margins increase the dose to the normal tissues.
As a consequence of the anatomical conditions around the bladder it is reasonable to assume that the bladder dimensions do not grow in a uniform way during bladder filling but it is expected that the caudal part of the bladder is more stable than the cranial part as well as the other dimensions are dependent on the tension of the pelvic muscles and intestinal peristaltic movements. Additionally, the tumor may alter the normal physiological stretch pattern of the bladder. Therefore, in our analysis an assumption was made that as a worst case scenario the bladder grows only in one direction and the other two directions remain unchanged. In reality this is not always the case and it is, of course, possible that one of the directions is even subject to decrease, i.e. because of the peristaltic movement while the other dimensions increase more than what is expected from the modeled volume change.
Intrafractional margins for the treatment of the bladder have been previously investigated based on CBCT imaging before and after treatment. A recent study calculated margins to account for intrafractional filling of the bladder, which were isotropic with a magnitude of 0.58 cm due to the definition of the coordinate system [Citation15]. The intrafractional margins are dependent on the selected technique of CBCT image registration before irradiation, and in our method soft-tissue matching is used which takes the daily bladder position variations into account. Our approach to define the growth of the bladder dimensions along three perpendicular axis is more simple than a proper 3D analysis of treatment margins. In addition, the ellipsoidal model does not take possible rotations of the bladder into account. However, the simple ellipsoidal model gives a reasonable accuracy for estimation of bladder volume and rate of change of the bladder largest dimension as a function of bladder volume. Therefore, intrafractional margins derived from the ellipsoidal main axis measurements are safe in our set-up with 15 minute time interval between planning CTs and < 15 minutes approximate time from CBCT to the end of a single treatment fraction, provided that patient positioning is corrected by soft-tissue matching before irradiation. According to the observed changes of bladder maximum dimensions along three orthogonal axis were all smaller than 10 mm. This is rather well in accordance with the research by Foroudi et al. [Citation15], where a uniform 0.58 cm margin, giving 1.16 cm when combined for the whole bladder diameter in order to be comparable with our result, was reported to cover the intrafractional filling of the bladder for 90% of the patients.
Since the actual rate of urine formation varies between patients and even in an individual as a function of time it is important to aim at more effective hydration during planning CTs than during treatment. The time of the image analysis and irradiation after online CBCT should be kept less than or equal to the time interval between the successive CT:s. In addition, it is probably useful to have several planning CTs instead of two extreme conditions (empty and full bladder) to get more detailed modeling of geometrical changes in pelvic area. Our planning CT protocol represents the worst case of bladder filling during a 15 minute interval. Nevertheless, on two treatment fractions of 25 the bladder filling rate during treatment was clearly higher than during planning CT. One possible explanation for this could be potential excess hydration during chemotherapy.
In our clinic a method for minimizing the CBCT imaging dose was adopted in ART for bladder. Using manufacturer CBCT imaging protocols in XVI, the maximum measured dose in the PMMA phantom was 35.7 mGy per image, which adds up to a maximum cumulative dose of 1.1 Gy in 32 fractions for the whole pelvic area. The additional dose is distributed to the small bowel located within the high dose region as well as rectum, which could theoretically even lead to exceeding the dose limits for these OARs. By optimization of CBCT imaging protocols, in the best case scenario the maximum cumulative dose in 32 fractions could be lowered down to 0.14 Gy. However, while optimizing the dose it is crucial to keep image quality at sufficient level to be able to reliably recognize the bladder anatomy. Another source of additional dose to the pelvic area is the repeated treatment planning CT imaging, which typically causes a weighted dose less than 20 mGy per scan (measured in a head phantom). Drops of Lipiodol in bladder wall generated artefacts in CBCT images, which seemed to diminish during the course of treatment as some of the Lipiodol volume was diffused in the surrounding tissues.
In conclusion, the use of Lipiodol contrast agent enables partial bladder treatment with reduced irradiation of the healthy bladder volume. The ellipsoidal bladder model can be used for the estimation of the bladder volume changes and the upper limit of the bladder wall movement during the treatment fraction.
Declaration of interest: The first contributing author (J.K.) holds a research grant from Elekta Limited with permission to publish.
References
- Muren LP, Smaaland R, Dahl O. Organ motion, set-up variation and treatment margins in radical radiotherapy of urinary bladder cancer. Radiother Oncol 2003;69:291–304.
- Meijer GJ, Rasch C, Remeijer P, Lebesque JV. Three-dimensional analysis of delineation errors, setup errors, and organ motion during radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys 2003;55:1277–87.
- Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose–volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76:S101–7.
- Redpath AT, Muren LP. CT-guided intensity-modulated radiotherapy for bladder cancer: Isocentre shifts, margins and their impact on target dose. Radiother Oncol 2006;81: 276–83.
- Burridge N, Amer A, Marchant T, Sykes J, Stratford J, Henry A, et al. Online adaptive radiotherapy of the bladder: Small bowel irradiated-volume reduction. Int J Radiat Oncol Biol Phys 2006;66:892–7.
- Pos F, Bex A, Dees-Ribbers HM, Betgen A, van Herk M, Remeijer P. Lipiodol injection for target volume delineation and image guidance during radiotherapy for bladder cancer. Radiother Oncol 2009;93:364–7.
- Søndergaard J, Olsen KØ, Muren LP, Elstrøm UV, Grau C, Høyer M. A study of image-guided radiotherapy of bladder cancer based on lipiodol injection in the bladder wall. Acta Oncol 2010;49:1109–15.
- Foroudi F, Wong J, Haworth A, Baille A, McAlpine J, Rolfo A, et al. Offline adaptive radiotherapy for bladder cancer using cone beam computed tomography. J Med Imaging Radiat Oncol 2009;53:226–33.
- Vestergaard A, Søndergaard J, Petersen J, Høyer M, Muren LP. A comparison of three different adaptive strategies in image-guided radiotherapy of bladder cancer. Acta Oncol 2010;49:1069–76.
- Tuomikoski L, Collan J, Keyriläinen J, Visapää H, Saarilahti K, Tenhunen M. Adaptive radiotherapy in muscle invasive urinary bladder cancer – An effective method to reduce the irradiated bowel volume. Radiother Oncol 2011;99:61–6.
- Meijer GJ, van der Toorn P, Bal M, Schuring D, Weterings J, de Wildt M. High precision bladder cancer irradiation by integrating a library planning procedure of 6 prospectively generated SIB IMRT plans with image guidance using lipiodol markers. Radiother Oncol 2012;105:174–9.
- Lalondrelle S, Huddart R, Warren-Oseni K, Hansen VN, McNair H, Thomas K, et al. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys 2011;79:705–12.
- Foroudi F, Wong J, Kron T, Rolfo A, Haworth A, Roxby P, et al. Online adaptive radiotherapy for muscle-invasive bladder cancer: Results of a pilot study. Int J Radiat Oncol Biol Phys 2011;81:765–71.
- Korhonen J. Using volumetric-modulated arc therapy and cone-beam computed tomography image guidance with six degrees of freedom in patient positioning for the radiation therapy of patients with bladder cancer. Aalto University, School of Electrical engineering, Helsinki, Finland. Master's Thesis http://urn.fi/URN:NBN:fi:aalto-201207022699; 2011.
- Foroudi F, Pham D, Bressel M, Gill S, Kron T. Intrafraction bladder motion in radiation therapy estimated from pretreatment and posttreatment volumetric imaging. Int J Radiat Oncol Biol Phys 2013;86:77–82.