Abstract
In 2003, the Danish Breast Cancer Cooperative Group (DBCG) initiated DBCG-IMN, a prospective study on the effect of adjuvant internal mammary lymph node radiotherapy (IMN-RT) in patients with early lymph node positive breast cancer (BC). In the study, standard DBCG IMN-RT was provided only to patients with right-sided BC. We provide estimates of doses to IMNs and organs at risk (OARs) in patients treated with the non-CT-based RT techniques used during the DBCG-IMN study. Material and methods. Five DBCG RT regimens were simulated on planning CT scans from 50 consecutively scanned BC patients, 10 in each group. Intended target volumes were chest wall or breast and regional lymph nodes ± IMNs. Field planning was conducted in the EclipseTM RT treatment planning system. Subsequently, IMN clinical target volumes (CTVs) and OARs were delineated. Estimates on doses to the IMN-CTV and OARs were made. Results. IMN dose coverage estimates were consistently higher in right-sided techniques where IMN treatment was intended (p < 0.0001). Estimated doses to cardiac structures were low regardless of whether IMNs were treated or not. Post-lumpectomy patients had the highest estimated lung doses. Conclusion. Overall, simulator-based treatment using the DBCG RT techniques resulted in satisfactory coverage of IMNs and acceptable levels of OAR irradiation.
Adjuvant radiotherapy (RT) to the internal mammary lymph nodes (IMNs) is a controversial subject. In early breast cancer (BC), the probability of metastatic involvement of the IMNs rises with increasing tumor size, medial tumor location and number of axillary lymph nodes with metastases [Citation1]. Metastasis to the IMNs has been shown to be as detrimental to prognosis as axillary lymph node metastasis [Citation2]. Studies on the use of IMN dissection have shown no benefit [Citation3]. Adjuvant RT to the mammary region and regional lymph nodes was documented to increase survival in patients with early BC, however, the benefit of RT to individual lymph node stations is still unknown [Citation4–6]. The effect of RT to the IMNs has been investigated mainly in retrospective studies [Citation7,Citation8]. Results are divergent, perhaps due to the methodological limitations of the studies. One randomized prospective study on the effect of IMN-RT including 270 patients and with a median follow-up time of 2.7 years showed no increase in relapse-free survival [Citation9]. Studies of larger proportions would be needed to have power to detect a potential survival advantage of IMN-RT. The European Organisation for Research and Treatment of Cancer (EORTC) trial 22922/10925 randomized 4004 patients to ± medial supra-clavicular/IMN-RT [Citation10]. Results are pending.
In 2003, the Danish Breast Cancer Cooperative Group (DBCG) initiated DBCG-IMN, a prospective study on the effect of adjuvant IMN-RT in patients with early lymph node positive BC. At the time, awareness of the potential cadiotoxic effects of RT, especially in combination with anthracyclines and trastuzumab, was growing. A possible beneficial effect of IMN-RT, of which there was no evidence, was likely to be cancelled out by cardiac morbidity, especially in patients with left-sided BC. Consequently, it was decided that patients with right-sided, lymph node positive BC would receive RT to breast/chest wall and regional lymph nodes including the IMN, while in patients with left-sided BC, the IMNs would no longer be a target for adjuvant RT [Citation11].
In the early 2000s, techniques for delivery of adjuvant RT in BC were changing rapidly in the RT departments treating the Danish BC patients. Studies on the previously applied standard DBCG techniques had pointed out that especially in post-lumpectomy (PL) patients receiving wide tangential fields, the IMNs were not always sufficiently covered [Citation12]. Furthermore, this technique sometimes resulted in inclusion of large volumes of ipsilateral lung, heart and even contralateral breast [Citation11]. As a consequence, the introduction of three dimensional CT-guided (3D-CT-guided) dose-planning in the PL setting was prioritized. summarizes the implementation of DBCG-IMN guidelines, the non-CT/simulator-based techniques used for IMN-RT and the transition from simulator-based to 3D-CT-guided dose planning in the DBCG RT departments. The aim of the current study is to perform quality assurance on the simulator-based IMN-RT in the DBCG-IMN study by: 1) estimating IMN dose coverage both in right-sided patients, where IMN-treatment was intended, and in left-sided patients, in whom no IMN treatment was intended; and 2) estimating doses to organs at risk (OARs) to evaluate the risk of toxicity in right- and left-sided patients.
Table I. Overview of the implementation of DBCG-IMN radiotherapy guidelines, non-CT-based techniques for IMN radiotherapy and the transition from simulator-based to 3D-CT-guided dose planning in the DBCG radiotherapy departments.
Material and methods
Patients
Planning CT scans from 50 BC patients (20 PM and 30 PL patients) treated with adjuvant RT at the Department of Clinical Oncology in Aarhus, Denmark, in the period from November 2010 to January 2011 were used for the reconstruction of five simulator-based RT techniques used after implementation of the DBCG-IMN RT guidelines. Selection of patients was based solely on side and type of operation. The 20 PM patients consisted of 10 consecutively scanned left-sided and 10 right-sided patients. Likewise, the 30 PL patients were consecutively scanned left-sided (n = 10) and right-sided (n = 20). CT images with 3 mm slice thickness were acquired from the sixth cervical vertebra to at least 5 cm caudally of the sulcus of the breast and including the lungs. Scans were not contrast enhanced. Patients were scanned in treatment position on a breast board with the ipsilateral arm abducted 90–110° and the head turned 10–20° to the contralateral side. The angle of the breast board was adjusted so that the patient's sternum was parallel to the table top. In all patients, radioopaque marker wires were placed on the skin to mark the palpated midline of the sternum and the medial edge of the ipsilateral sternocleidomastoid muscle. In PM patients, the mammary region was marked corresponding to the position of the contralateral infra-mammary fold. This was carried out with both arms raised symmetrically above the head. The marker wire was extended to the mid-axillary fold. In PL patients, the breast with a 1 cm margin was marked extending to the mid-axillary fold.
CT images were transferred to the EclipseTM (Varian Medical Systems, Palo Alto, CA, USA) treatment planning system. Patient surface and all wire markings were contoured. Four non-CT-based irradiation techniques, two left-sided and two right-sided, were reconstructed on separate groups of 10 patients by using the markings together with surface and bony anatomy on digitally reconstructed radiographs (DRRs). Thus, the field borders and beam arrangements were defined without delineated target volumes as was the normal procedure in the simulation era. One PL technique that had been predominantly applied using a simulator with CT option resulting in five transversal CT slices in the mammary region was also reconstructed in 10 patients. All reconstructions were carried out by one oncologist (LT) under guidance from two oncologists (BVO and MO) and one physicist (MST) who had all worked with the historical techniques when they were in daily clinical use.
Field reconstruction
For all patients, an anterior photon field angled 10–15° away from the spinal cord was used to treat axillary and periclavicular nodes. In PM patients, this field also covered the most lateral aspect of the chest wall. PM patients with right-sided BC received two electron fields to the chest wall and IMNs, respectively (PM Right), while PM patients with left-sided BC were treated with one electron field to the chest wall (PM Left). In PL patients with right-sided BC, 10 patients were planned using wide tangential fields (PL Right T) and in 10 additional patients, a separate IMN electron field plus tangential fields were employed for IMNs and the breast (PL Right E + T). PL patients with left-sided BC received tangential fields to the breast (PL Left). A number of subfields were utilized for tangential beams as needed to ensure a homogenous dose distribution. For a detailed description of field arrangements, see Supplementary Table I (Available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.813643.) and .
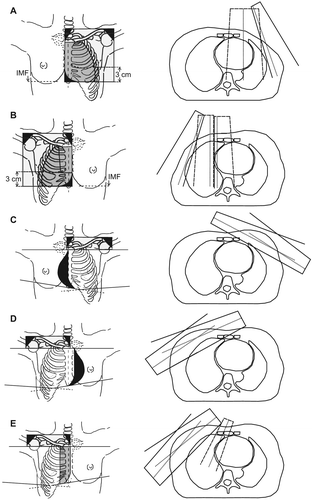
Figure 1. Field arrangements in the five radiotherapy techniques. Left: A: Left-sided two field arrangement in post-mastectomy patients (PM Left), B: Right-sided three field arrangement in post-mastectomy patients (PM Right), C: Left-sided standard tangents in post-lumpectomy patients (PL Left), D: Right-sided wide tangents in post-lumpectomy patients (PL Right T) and E: Right-sided electron field combined with tangential fields in post-lumpectomy patients (PL Right E + T). Black: Shielded areas. Light grey: Photon fields. Dark grey: Electron fields. Right: Axial slices: Dashed Lines: Electron field borders, Solid lines: Photon field borders. Dotted lines: Field axis.
Dose reconstruction
Dose distributions were calculated using the anisotropic analytical algorithm (AAA) for photons and the Monte Carlo electron algorithm for electrons. Plans were normalized to deliver 90% of the prescribed dose to the mid-axilla defined as a point at half the antero-posterior distance at the lower border of the humeral head. In PM patients, tissue depths measured on individual CT-images were used to choose electron energies. For details on measurements, see Supplementary Table I(Available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.813643.). Field weights were adjusted to deliver a median dose of 2 Gy per fraction (minimum 1.84 Gy and maximum 2.16 Gy) in the electron field(s). In PL patients, field weights were adjusted to provide a homogenous dose at the CT slice in the middle of the breast except for PL Right E+ T patients, where five CT slices in the mammary region were reviewed. In these five CT slices, the electron energy and bolus was chosen so the 90% isodose curve included the breast and regional lymph nodes. The prescribed dose was 48 Gy in 24 fractions, 5 fractions per week in all patients.
Contouring
After reconstruction of irradiation techniques, the IMN clinical target volume (CTV) including IMNs in the first four intercostal spaces, heart, left anterior descending coronary artery (LAD) and lungs were contoured in all patients by one oncologist (LT) as per the national DBCG guidelines [Citation13].
Statistical analysis
Dose-volume histograms (DVHs) were calculated for all delineated volumes. A point dose to one point at a location deemed to be in the IMN area in a CT-slice in intercostal spaces 1 and 3 was calculated for comparison purposes [Citation14]. The relative volume V irradiated to a minimum dose x, Vx, e.g. V40Gy for the heart, was determined from the DVH graph. Mean heart dose (MHD) was obtained from the DVH statistics.
Q-Q-plots were made on dose coverage estimates to confirm no major deviations from normality in the five patient groups. Means, standard deviations (SDs) and 95% confidence intervals (CIs) were calculated. After performing tests for unequal variances, unpaired Student's t-test with or without the assumption of equal variances was used as appropriate to compare mean IMN V43.2Gy in patients with right-sided contra patients with left-sided BC. Differences were considered statistically significant for p < 0.05.
Results
IMN dose estimates
Results are summarized in . shows individual V43.2 Gy values for the five techniques. For PM Right patients, the mean relative IMN volume irradiated with at least 43.2 Gy corresponding to 90% of the prescribed dose (IMN V43.2Gy) was 86.9% with a 95% CI of [82.0; 90.7]. For PM Left patients, the IMN V43.2Gy was 8.0% [−3.5; 19.5]. The difference was statistically significant (p < 0.0001). Likewise, PL Right T and PL Right E+ T patients had a better dose coverage of the IMN CTV than did PL Left patients with IMN V43.2Gy = 73.4% [56.8; 89.9] and 85.1% [82.0; 88.3] versus IMN V43.2Gy = 16.2% [0.9; 31.4]. Again, differences were statistically significant (p < 0.0001 for both). As can be seen from the confidence intervals, variation in dose coverage estimates was largest in techniques utilizing tangential fields for IMN treatment, namely PL Left and PL Right T. presents the individual IMN point doses in intercostal space three for the five techniques.
Figure 2. Internal mammary node dose coverage in the different techniques. Horizontal lines: Mean. Filled circles: individual patient dose coverage estimates.
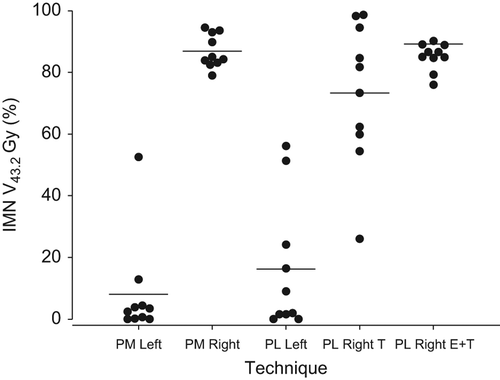
Figure 3. Internal mammary node point doses at a point within the internal mammary node clinical target volume contour in the third intercostal space.
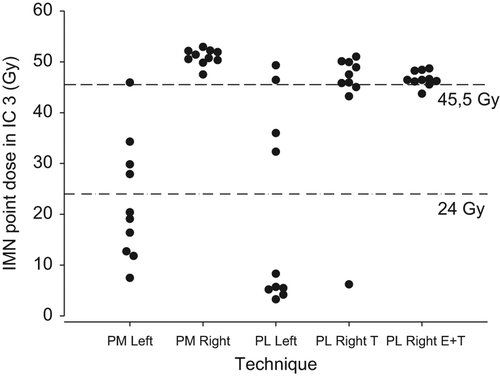
Table II. Summary of dose coverage estimates for internal mammary lymph nodes and organs at risk in the five standard DBCG techniques.
Cardiac dose estimates
Estimated average MHD varied depending on treatment technique, see . PM Left patients had an estimated mean MHD of 1.8 Gy [1.5; 2.0], while in PM Right patients, the estimated mean MHD was 3.5 Gy [2.5; 4.4]. PL Left patients had a mean MHD estimate of similar proportions: 3.4 Gy [2.5; 4.2]. Tangential fields for right-sided PL patients, PL Right T, resulted in the lowest estimated mean MHD, namely 0.8 Gy [0.7; 1.0], while PL Right E+ T resulted in a somewhat larger mean MHD estimate of 2.2 Gy [1.6;2.9]. PM Right patients had the largest estimated heart mean V20Gy of 4.7% [2.5; 6.9], while PL Left patients had the largest estimated heart mean V40Gy of 2.2% [1.1; 3.4].
LAD dose estimates
Average LAD mean dose (LADmean) estimates varied between treatment techniques (). Here, PL Left patients had the largest LADmean estimate of 21.6 Gy [10.2; 33.0], whereas PL Right T patients had the smallest estimate of 0.3 Gy [0.2; 0.4].
Pulmonary dose estimates
PM techniques resulted in comparable lung doses with an estimated mean ipsilateral lung V20Gy for PM Left of 17.1% [14.1; 20.1] and of 19.3% [17.3; 21.2] for PM Right. PL Left had a mean ipsilateral lung V20Gy of 19.2% [11.8; 26.7], while PL Right T and PL Right E+ T had a mean ipsilateral lung V20Gy estimates of 26.0% [19.1; 33.0] and 37.9% [33.8; 42.0], respectively.
Discussion
The current study documents the rapid changes occurring during the 2000s in the methods used to deliver adjuvant RT for Danish patients with early lymph node positive BC. The DBCG-IMN RT techniques were implemented in most DBCG RT departments within a few months after announcement of the guidelines. Especially in the PL setting, simulator-based treatments were swiftly replaced by 3D-CT-guided dose planning, and only a minor subset of PL patients participating in the DBCG-IMN study received non-CT-guided dose planning. Respiratory gating, which has been demonstrated to limit doses to OARs, was already introduced in one center at the initiation of DBCG-IMN, allowing IMN-RT for all PL patients [Citation15,Citation16]. In contrast, for PM patients the transition to 3D-CT-guided dose planning was made over a longer period.
For IMN-RT to be beneficial in terms of survival, the positive effects of IMN treatment on mortality from BC must outweigh the negative effects in terms of risk of cardiac and pulmonary disease and the risk of inducing second malignancies [Citation17]. It is not within the scope of the present study to evaluate the possible negative effects on cosmesis or other types of late radiation-induced morbidity with regional RT, but studies on patients treated with similar techniques found acceptable long-term adverse effects with RT techniques similar to those applied in the DBCG-IMN [Citation18,Citation19].
Estimates of IMN dose coverage expressed as V43.2Gy indicate that as intended, right-sided techniques in general provided larger doses to the IMNs than did left-sided techniques. Especially the techniques utilizing electrons for IMN coverage had a high degree of dose coverage with little variation in the patient population (). However, in techniques using tangential fields for target coverage, estimates on IMN dose coverage were more uncertain. For PL Right T, where IMN-RT was intended, the true mean IMN V43.2 Gy could with 95% confidence be as low as 56.8%, whereas for PL Left patients, where no IMN treatment was intended, the true mean IMN V43.2 Gy could be as high as 31.4%. This is in accordance with results from an earlier study on the use of tangential fields in non-CT-based treatment planning after lumpectomy. Here, none of nine patients receiving tangential fields after right-sided lumpectomy had IMNs plus 5 mm margin within the borders of the tangential fields [Citation12]. In the EORTC 22922/10925 individual case review on selected cases from 19 of 45 institutes, point doses at the estimated location of the IMNs in a cranial or a central CT slice from the treatment plan, whichever was deemed most relevant to the IMN target, were calculated for 45 patients in the treatment arm and for 42 patients in the control arm. For 98% of patients in the treatment arm, this resulted in point doses of at least 85% of the prescribed dose of 50 Gy, whereas in the control arm, 10% of patients had point doses of more than 50% of the prescribed dose [Citation14]. In the present study, it was attempted to investigate point doses in a similar manner. For each patient, a point dose at a location deemed to be in the IMN area in a CT-slice in intercostal spaces 1 (data not shown) and 3 was calculated (). Results were similar for both intercostal spaces. For the right-sided techniques, doses have virtually the same distribution as in the EORTC 22922/10925, but in general, higher doses are seen in the left-sided regimes. This shows that point doses as the only means of target coverage do not fully reflect the dose received by the entire target volume, but also indicates that doses in the “treatment arm” i.e. the right-sided regimes of the DBCG-IMN study may well have been comparable to those in the EORTC study. In the ideal setting in which to evaluate the effect of IMN RT, patients with right-sided BC would have had a relevant dose in terms of tumor control probability to the entire IMN CTV, and patients with left-sided BC would have had no dose to the IMN CTV. This is however not possible, not even with today's more advanced techniques, as the IMNs are situated close to the breast/chest wall. We found that in right-sided PL patients receiving tangential fields, some patients are likely to have received lower than intended IMN doses, whereas left-sided PL patients may in rare cases have received IMN doses of clinical relevance. This could slightly diminish the power of the DBCG-IMN study to detect a true effect of IMN treatment. However, only two departments used the simulator-based PL Right T technique for a limited time. The vast majority of right-sided patients in the study receiving non-CT-based treatment would have had IMN treatment with electron fields, with the techniques shown to deliver high IMN dose coverage. Moreover, doses delivered to the IMNs in left-sided PM patients were limited.
Pulmonary dose estimates revealed that in PM patients, V20Gy to the ipsilateral lung were similar for right- and left-sided patients with 17.1% [14.1; 20.1] versus 19.3% [17.1; 21.2]. In another study on PM RT investigating the same techniques on patients with large variations in anatomy, V20Gy was found to be in the range of 15.4–24.8%, agreeing with our results [Citation20]. In the present study the central lung distance (CLD), which has been shown to correlate with V20Gy was used in planning as a means to limit the dose to the ipsilateral lung [Citation21]. Arthur et al., using a PL treatment technique of partial wide tangents very similar to the PL Right T technique in five patients, but prescribing 50 Gy in 25 fractions, found ipsilateral V20Gy values in the range of 25–35% for both right- and left-sided treatments, which is in concordance with the 26% [19.1; 33.0] found in the present study for PL Right T patients. In the same study, standard tangential fields with lateral and medial field borders placed 1.5 cm from palpated breast tissue, i.e. resulting in shallower tangents than in the present study, had V20 Gy estimates ranging from 12–20%, which as expected is less than our 30.4% [22.2; 38.7] [Citation22]. For the PL Right E+ T technique, V20 Gy estimates ranged from 31.5% to 47.4%. The combination of an anterior electron field and tangential fields for the breast resulted in lung doses that might entail some risk of toxicity. Indeed, in the EORTC 22922/10925 trial, where a similar technique was used for IMN treatment, an increase in lung complications has been observed at three years, but these complications had no impact on patient performance status [Citation23].
In general, estimates of mean MHD in the present study were low, ranging from 0.8 Gy to 3.5 Gy in all patient groups. Heart V20 and V40 values in the PM Right group were comparable to those reported by Thomsen et al. in an investigation of this technique [Citation20]. Taylor et al, reconstructing historically used Danish BC RT techniques in a single patient of typical anatomy in general reported larger estimates of both MHD and mean doses to the LAD than those found in the present study [Citation24]. For instance, with right-sided wide tangents plus an electron scar boost, as in the PL Right technique, a MHD of 3.3 Gy was found with an LAD mean dose of 1.9 Gy. Also, for left-sided PL patients treated with tangential fields to the breast and an electron or tangential scar boost, the estimated MHD was as high as 6.1 and 6.3 Gy. This may be explained by differences in patient positioning (flat) and anatomy, lack of adjustment of field borders in case of CLD above 30 mm, or in the doses prescribed. It seems unlikely that boost doses could account for the differences. Indeed, boost dose contribution to MHDs even in PM patients was found to be less than 1 Gy [Citation24].
Overall, we found that both mean MHD estimates and average LAD mean dose estimates differed in size depending on RT technique, although the two did not seem to co-variate. This illustrates the impact of specific field arrangements on doses to substructures in the heart. In an earlier DBCG study randomizing PM patients to ± RT including the IMNs, no excess mortality from ischemic heart disease was found at 10 years median follow-up [Citation25]. Patients were treated with field arrangements similar to the ones used in our study, but with IMN-RT for all, most likely resulting in larger MHDs than in our study. If MHD is considered to be correlated with an increased risk of cardiac toxicity, even at the dose levels encountered here, we would expect cardiac complications to occur at comparable rates with right- and left-sided treatment techniques in the DBCG-IMN study [Citation26,Citation27].
Reconstruction as a means of dose estimation
The use of modern techniques to derive estimates of doses to target tissues and OARs in patient cohorts treated in the past is an attractive method in that it allows assessment of risks and benefits from treatments previously applied [Citation24]. In the present study, CT-scans from consecutively scanned patients selected solely on the basis of side and type of operation were used to re-plan well-described techniques. Modern dose calculation algorithms, AAA and the Monte Carlo electron algorithm that provide realistic dose estimates in volumes close to lung tissue were used. Nevertheless, care must be taken not just to take these estimates at face value, but to also consider the limitations of the method.
Several factors may contribute to an underestimation of the actual variation in IMN dose coverage. Historical RT techniques were applied on new planning CT scans in one department. Multiple patients were used in the reconstructions to gain knowledge of the effect of anatomical variations between patients on target and OAR dose estimates. Although techniques were well described, execution of the guidelines might still in practice have been subject to some minor local variation. All plans were reconstructed by one oncologist, eliminating variability between individual planners. Also, dose calculations were carried out using equipment specifications from one department only. Possible set up variations during treatment courses were unaccounted for.
Systematic errors in estimation of IMN dose coverage could be introduced in a number of ways. Boost irradiation was not taken into account in the present study, which might have led to underestimation of IMN doses. In some departments, patients were treated with both arms elevated. Patients in the current study were scanned with only the ipsilateral arm elevated. However, the position of the IMNs has been found to depend little on arm position, and so impact on IMN doses is expected to be minimal [Citation28].
Finally, the equipment used in dose planning was different from that used historically, and some compromises had to be made. In the past, wedges were used for PL plans to ensure dose homogeneity in the breast, but due to equipment limitations, a number of subfields had to be applied instead. This was done by adding subfields to the tangential main fields achieving a stepwise simulation of a wedge, while looking at dose distribution in only one central plane of the breast, as this was the way wedge angles were originally chosen.
Conclusion
In the DBCG-IMN study, non-CT-based techniques using electrons for IMN treatment provided excellent dose coverage with little variation between patients. The less frequently used tangential fields proved to deliver more variable doses. IMN doses in left-sided techniques were modest. Estimated doses to cardiac structures were low regardless of whether IMNs were treated or not. PL patients had the highest estimated lung doses. Overall, simulator-based treatment using the DBCG RT techniques resulted in satisfactory coverage of IMNs and acceptable levels of OAR irradiation.
Supplementary Table I
Download PDF (1.6 MB)Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by Aarhus University Hospital, the Danish Cancer Society, Breast Friends and CIRRO – the Lundbeck Foundation Center for Interventional Research in Radiation Oncology.
References
- Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat 2008;107:379–87.
- Dahl-Iversen E, Tobiassen T. Radical mastectomy with parasternal and supraclavicular dissection for mammary carcinoma. Ann Surg 1969;170:889–91.
- Meier P, Ferguson DJ, Karrison T. A controlled trial of extended radical versus radical mastectomy. Ten-year results. Cancer 1989;63:188–95.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–55.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106.
- Arriagada R, Le MG, Mouriesse H, Fontaine F, Dewar J, Rochard F, et al. Long-term effect of internal mammary chain treatment. Results of a multivariate analysis of 1195 patients with operable breast cancer and positive axillary nodes. Radiother Oncol 1988;11:213–22.
- Olson RA, Woods R, Speers C, Lau J, Lo A, Truong PT, et al. Does the intent to irradiate the internal mammary nodes impact survival in women with breast cancer? A population-based analysis in British Columbia. Int J Radiat Oncol Biol Phys 2012;83:e35–e41.
- Kaija H, Maunu P. Tangential breast irradiation with or without internal mammary chain irradiation: results of a randomized trial. Radiother Oncol 1995;36:172–6.
- Musat E, Poortmans P, Van den Bogaert W, Struikmans H, Fourquet A, Bartelink H, et al. Quality assurance in breast cancer: EORTC experiences in the phase III trial on irradiation of the internal mammary nodes. Eur J Cancer 2007;43:718–24.
- Overgaard M, Christensen JJ. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol 2008;47:639–53.
- Nielsen HM, Christensen JJ, Aagaard T, Thingholm J, Overgaard M, Grau C. A simple method to test if the internal mammary lymph nodes are covered by the wide tangent technique in radiotherapy for high-risk breast cancer. Clin Oncol (R Coll Radiol) 2003;15:17–24.
- Nielsen MH, Berg M, Pedersen AN, Andersen K, Glavicic V, Jakobsen EH, et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: National guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol 2013.
- Poortmans P, Kouloulias VE, Venselaar JL, Struikmans H, Davis JB, Huyskens D, et al. Quality assurance of EORTC trial 22922/10925 investigating the role of internal mammary– medial supraclavicular irradiation in stage I–III breast cancer: the individual case review. Eur J Cancer 2003;39:2035–42.
- Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol 2011;50:42–50.
- Johansen S, Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI, Olsen DR. Dose evaluation and risk estimation for secondary cancer in contralateral breast and a study of correlation between thorax shape and dose to organs at risk following tangentially breast irradiation during deep inspiration breath-hold and free breathing. Acta Oncol 2011; 50:563–8.
- Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother Oncol 2013;106:42–9.
- Lundstedt D, Gustafsson M, Steineck G, Alsadius D, Sundberg A, Wilderang U, et al. Long-term symptoms after radiotherapy of supraclavicular lymph nodes in breast cancer patients. Radiother Oncol 2012;103:155–60.
- Lyngholm CD, Christiansen PM, Damsgaard TE, Overgaard J. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol 2013;52:259–69.
- Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M, Ewertz M, et al. Post-mastectomy radiotherapy in Denmark: from 2D to 3D treatment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncol 2008;47:654–61.
- Das IJ, Andrews JZ, Cao M, Johnstone PA. Correlation of 2D parameters to lung and heart dose-volume in radiation treatment of breast cancer. Acta Oncol 2013;52:178–83.
- Arthur DW, Arnfield MR, Warwicke LA, Morris MM, Zwicker RD. Internal mammary node coverage: an investigation of presently accepted techniques. Int J Radiat Oncol Biol Phys 2000;48:139–46.
- Matzinger O, Heimsoth I, Poortmans P, Collette L, Struikmans H, Van den Bogaert W, et al. Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol 2010;49:24–34.
- Taylor CW, Bronnum D, Darby SC, Gagliardi G, Hall P, Jensen MB, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol 2011;100:176–83.
- Hojris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet 1999;354:1425–30.
- Andratschke N, Maurer J, Molls M, Trott KR. Late radiation-induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol 2011;100:160–6.
- McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167–75.
- Dijkema IM, Hofman P, Raaijmakers CP, Lagendijk JJ, Battermann JJ, Hillen B. Loco-regional conformal radiotherapy of the breast: delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol 2004;71:287–95.
