Abstract
Gastroesophageal cancers are heterogeneous diseases with a poor outcome. Prognostic and predictive factors are needed to improve patient survival. Hypoxia is an adverse prognostic factor and is associated with resistance to chemo- and radiotherapy in various cancers. However, knowledge on the impact of hypoxia in gastroesophageal cancer is limited. The aim of this study was to evaluate potential prognostic factors in terms of a subset of hypoxia-responsive genes and clinicopathological parameters in patients with gastroesophageal cancer. Material and methods. Ninety-five patients with loco-regional gastroesophageal cancer treated with curative intent were retrospectively analyzed. Based on formalin-fixed paraffin-embedded diagnostic biopsies gene expressions of 15 hypoxia-induced and pH-independent genes from a previously described hypoxia gene expression classifier was quantified. The prognostic impact was evaluated for overall survival (OS) and disease-specific survival (DSS). Uni- and multivariate Cox proportional hazards model was used to identify hypoxia-responsive gene expression and clinicopathological parameters as prognostic markers. Results. An unsupervised hierarchical clustering of hypoxia regulated genes showed two well-differentiated patient clusters: One cluster of tumors with high gene expression and another with low gene expression, indicating a more hypoxic genotype versus a less hypoxic genotype respectively. As the group of esophageal squamous cell carcinomas (ESCC) alone showed intra-group heterogeneity this group was ranked according to the gene expression of the 15 genes. The most hypoxic third showed a trend towards a poorer outcome in terms of OS [HR = 0.48 (CI 0.21–1.07), p = 0.07] and DSS [HR = 0.48 (CI 0.18–1.24), p = 0.13]. Treatment response was identified as an independent prognostic factor for DSS in the group of ESCC [HR = 0.21 (CI 0.05–0.95), p = 0.04]. Conclusion. Gene expression analysis of 15 hypoxia-responsive genes was identified as a promising prognostic marker in patients with ESCC. Further studies confirming these results in larger patient cohorts are needed.
Esophageal cancer (EC), esophagogastric-junction cancer (EGJ) and gastric cancer (GC) are malignancies with a poor prognosis with a five-year overall survival ranging from 15% to 25% [Citation1,Citation2]. Patients presenting locally advanced, but potentially curable, disease at time of diagnosis need multimodality therapy in order to downstage the tumor, and thereby increase resectability rates, eliminate micrometastasis and prolong survival. Unfortunately, response rates to conventional therapeutic regiments vary between patients when treated identically. Hence, prognostic and predictive factors are needed to allow a tailored multimodality approach with increased efficacy.
Clinicopathological findings such as low cancer stage, pathological response and completeness of resection are recognized as prognostic factors in gastroesophagal cancers. Over recent years, molecular biomarkers have been identified as prognostic (e.g. EGFR/HER2, VEGF, p53, Bcl-X, E-cadherin) [Citation3,Citation4] and gene expression profiling has been associated with sensitivity to treatment and survival in gastroesophageal cancers [Citation5,Citation6].
Hypoxia is a hallmark of tumor formation and has been acknowledged to be an adverse prognostic factor in various cancers [Citation7]. Intra-tumoral hypoxia is associated with an aggressive phenotype, resistance to chemo- and radiotherapy and decreased overall survival [Citation8–10]. In addition, tumors are characterized by both diffusion limited and perfusion limited hypoxia and the hypoxic response leads to changes in expression of genes involved in tumor cell proliferation, angiogenesis and survival [Citation7,Citation11].
Hypoxia-induced radioresistance is a major obstacle in the therapeutic management of cancers, but can be counteracted with hypoxia-targeted therapy in some cancer sites [Citation12]. Thus, identification of hypoxia is of major interest and has been studied using oxygen sensitive electrodes, exogenous and endogenous hypoxic cell markers, particular in relation to radiotherapy [Citation13–16]. In addition, gene expression profiling is a promising approach to evaluate the hypoxic impact on the biological behavior [Citation17]. Based on in vitro, in silico and in vivo-derived studies on hypoxia responsive genes a few hypoxia gene expression signatures have been generated. These metagenes have proven successful in hypoxic classification and have shown to be of prognostic relevance in head and neck, breast, cervical and lung cancer [Citation18–20]. Recently, a 15-gene hypoxia gene expression classifier has been developed [Citation21,Citation22]. In contrast to other gene hypoxia signatures this metagene consists of 15 hypoxia-induced and pH-independent genes that were specifically identified based on in vitro [Citation23], in vivo and known tumor oxygenation status and showed to be of both prognostic and predictive impact for the hypoxia-modifying therapy, nimorazole, in patients with head and neck squamous cell carcinoma (HNSCC).
Gastroesophageal cancers have been subjected to only few hypoxia-related studies and current knowledge about the clinical significance of hypoxia in these malignancies is limited. Nonetheless, the high failure rate seen in gastroesophageal cancer after chemo- or chemoradiotherapy might be hypoxia- related.
The aim of the present study was to evaluate the 15 hypoxia responsive genes, clinicopathological parameters and therapy related variables as predictors for survival in patients with loco-regional gastroesophagal cancer.
Material and methods
Patients and treatment
Ninety-five patients with histological confirmed loco-regional EC, EGJ and GC treated with curative intent in the time period 1994–2004 and 2008–2011 were retrospectively analyzed.
Patient medical records were reviewed and information on clinicopathological parameters was placed into a research database after approval by the national ethics committees. The study was performed in accordance with the Declaration of Helsinki.
All patients underwent pretreatment diagnostic workup with physical examination, routine hematologic tests, upper gastrointestinal endoscopy with histopathological diagnostic biopsy confirming carcinoma, computed tomography (CT) of the chest and upper abdomen, pulmonary-function tests and in some cases staging by transesophageal endoscopic ultrasonography (EUS). Selected patients underwent laparoscopic ultrasonography (LUS) and patients included since 2010 underwent diagnostic FDG positron emission tomography-computed tomography (PET-CT). Preoperative staging was performed according to the TNM classification of the Union of International Cancer Control 4th–6th edition.
Patients with EC had received either preoperative chemoradiotherapy with concomitant 5-fluorouracil and 45 Gy in 25 fractions (fx), 5 fx/wk in a phase II protocol (1994–2004, n = 30) or preoperative concomitant cisplatin, 5-fluorouracil and 45 Gy in 25 fx, 5 fx/wk (2008–2011, n = 26). All patients with EGJ and GC had undergone treatment with curative intent with perioperative chemotherapy ad modem MAGIC (three cycles of epirubicin, cisplatin and capecitabine before and after surgery) (2009–2011, n = 39). After induction therapy a period of two to four weeks was mandated before surgery. This period included evaluation of resectability with a CT scan and in some cases gastrointestinal endoscopy or a magnetic resonance imaging (MRI) scan.
Based on diagnostic biopsies and tumor site, patients were characterized as either the EC or EGJ/GC group. In the EC group 93% of patients were diagnosed with squamous cell carcinomas (SCC) and 7% with undifferentiated carcinoma. This group will from here on be referred to as esophageal squamous cell carcinomas (ESCC). Eighty-two percent of patients in the EGJ/GC group had histologically proven adenocarcinomas (AC) and the remaining patients were diagnosed with adenosquamous carcinoma, signet ring cell carcinoma and unspecified carcinoma.
Treatment response evaluation and follow-up
In order to grade the response to therapy assessment of pathological response or radiographic response was performed. Pathological response was defined as either pathological complete response after neoadjuvant chemoradiotherapy or chemotherapy (ypCR) with no evidence of vital residual tumor remaining in the resected specimen or non-pathological complete response (non-ypCR) with presence of vital tumor. Radiographic complete response was defined as disappearance of all target lesions measured according to the RECIST-criteria, version 1.0. Radiographic response was only used as response evaluation to treatment in patients not undergoing surgery.
Classification of surgical resection was defined as ypR0 with more than 1 mm to resection margins, ypR1 with presence of vital tumor cells within 1 mm of the resection margin (microscopically positive) and ypR2 with macroscopic residual tumor. Pathological response evaluation and resection classification were carried out by experienced pathologists subspecialized in gastrointestinal pathology.
Patients treated in the phase II protocol were seen every three months until five years after treatment. All other patients were seen only during the first year after treatment completion at one, three, five and 12 months. Disease recurrence was evaluated with CT only when recurrence was suspected. All patients were observed for at least 18 months or until death. The last day of follow-up was on 22 March 2013.
Assessment of tumor hypoxia
Hypoxia was evaluated using gene expression on 15 hypoxia-induced and pH-independent genes derived from the previously described hypoxia gene expression classifier by Toustup et al. [Citation21]. Formalin-fixed, paraffin-embedded (FFPE) diagnostic, pretreatment tumor biopsies were used and presence of invasive carcinoma was verified on Hematoxylin-Eosin stained section. Due to insufficient amounts of RNA, gene expression analysis was carried out in 89 of 95 patients only. Briefly, total RNA was extracted from 7 μm FFPE-sections with a silica bead-based, fully automated isolation method for RNA in a robotic Tissue Preparation System using VERSANT Tissue Preparation Reagents (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). cDNA was synthesized using the High Capacity cDNA Archive kit and pre-amplified using the TaqMan PreAmp Master Mix Kit (Applied Biosystems; ABI). Quantitative real-time polymerase chain reaction (PCR) was performed on an ABI Prism 7900 HT Sequence Detector sing TaqMan Gene Expression PCR mastermix (Applied Biosystems; ABI). Data analysis was carried out using RealTime Statminer (Intergromics). ΔCt values were generated by normalizing to the geometric mean of the three reference genes RPL37A, ACTR3 and NDFIP1. Measurements were dismissed if Ct values were above 35 or if SD were more than 0.3. Probe sets used are listed in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.818247).
Statistical analysis
Potential clinicopathological prognostic factors, including age, gender, tumor characteristics and treatment response were evaluated using univariate Cox proportional hazards model and Cox multivariate proportional-hazard analysis. Survival analysis was carried out with the Kaplan-Meier method. In addition, survival data was expressed as hazard ratios (HR) using a univariate Cox proportional hazards model. Survival was calculated from the date of diagnosis (histologically confirmed) until death or last day of follow-up. Overall survival (OS) was defined as death from any cause and disease-specific survival (DSS) was defined as death from or with the primary gastroesophageal cancer. Statistical analysis was performed with STATA, Version 12. All p-values are two-sided with a 5% level of significance. HRs are presented with 95% confidence interval (CI).
Patient samples were first ranked for each of the 15 hypoxia-induced genes and the final rank was the median of the individual gene ranks. Each gene was given the same discriminating weight as opposed to the hypoxia gene expression signature in which genes were given slightly different weights, for details see Toustrup et al. [Citation21]. Subsequently, tumors were divided into tertiles and correlated with OS, DSS and treatment response. As the middle and the lower tertiles were overlapping these two tertiles were combined in the statistical analysis. Survival and multivariate analysis were performed as described above. Correlation with treatment response was carried out by using the χ2 statistical test. For gene clustering analysis, data were analyzed with Gene Cluster (version 3.0) using Pearson's correlation and complete linkage, and visualized using Treeview (Version 1.1.6r2)
Results
Patient and tumor characteristics
As patient and tumor characteristics were well balanced between the two ESCC populations the groups were analyzed together. Baseline characteristics are presented in . Seventy-five percent of patients in the ESCC group were male whereas 85% were males in the EGJ/GC group. Median age was 63 years and 62 years in the ESCC and EGJ/GC group, respectively. In both groups the median tumor length was 50 mm. Clinical T3 (cT3) stage was the predominant stage in the ESCC group with 75% whereas cT2 was the most frequent stage with 39% of patients in the EGJ/GC group. The clinical stage T3N1M0 was the predominant stage represented in the ESCC group with 35 cases. In the EGJ/GC group cT2N1M0 and cT3N1M0 was among the most frequent stages and were encountered in 10 and nine patients, respectively. One patient was staged T1N0M0 in the ESCC group.
Table I. Pretreatment patient and tumor characteristics.
Overall compliance to treatment was high in the ESCC group, but low in the EGJ/GC group. Among 56 patients in the ESCC group 47 patients (84%) completed all five cycles of preoperative chemotherapy and concomitant radiotherapy. In the EGJ/GC group 28 patients (72%) were able to complete three cycles of preoperative chemotherapy and only seven patients (18%) completed full course, including surgery and three cycles of post-operative chemotherapy. Reasons for not completing treatment were toxicity, death during treatment and post-operative complications.
Outcome analysis
The median follow-up was 29 months (range 3–175) in the ESCC group and 20 months (range 4–38) in the EGJ/GC group. The overall one- and three-year survival rates were 77% (CI 63–86%) and 51% (CI 37–63%) in the multimodality group of ESCC versus 77% (CI 60–87) and 49% (CI 30–65) EGJ/GC group.
Thirty-six patients (64%) had died in the ESCC group, 25 (69%) from ESCC, two from a second primary cancer (lung cancer), two from complications to treatment and seven from other causes. In the EGJ/GC group 18 patients (46%) had died, 14 (78%) from EGJ/GC, one from complications to treatment and three from other causes.
In the ESCC group 46 patients (82%) underwent surgery as compared to 34 patients (87%) in the EGJ/GC group. Reasons for not undergoing surgery were disease progression (seven patients in the ESCC group and five in the EGJ/GC group), death before surgery (two in the ESCC group) and patient's decision (one in the ESCC group).
Treatment response and classification of resection are presented in Supplementary Table II (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.818247). Pathological complete response was observed in resection specimens from 21 patients (37%) in the ESCC group. A radiographic complete response was not identified in any patients in this group. Treatment response was unknown in two patients in the ESCC group due to death during preoperative chemoradiotherapy or patient's request. In the EGJ/GC group all patients had a non-complete response to the preoperative chemotherapy.
An R0 resection was achieved in 43 of 46 patients (93%) who underwent surgery in the ESCC group whereas the remaining three patients (7%) had an R1 resection. In the EGJ/GC group 19 patients (56%) undergoing surgery had an R0 resection and 15 patients (44%) an R1 resection.
Hierarchical clustering of patients and genes
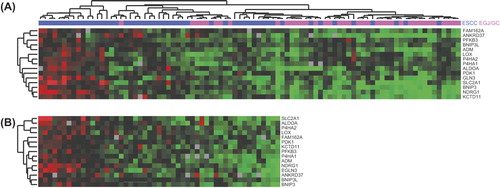
An unsupervised hierarchical clustering with the 15 genes could be performed on 89 patients. The analysis showed two well-differentiated patient clusters (). Up-regulated gene expression indicated a more hypoxic genotype and down-regulated gene expression a less hypoxic genotype. Inter-group heterogeneity between the ESCC and EGJ/GC group as well as intra-group heterogeneity in the ESCC group was observed. Thus, only the ESCC group consisted of patients with a more hypoxic genotype and of patients with a less hypoxic genotype. In contrast, all patients with EGJ/GC (adenocarcinoma) were characterized by reduced expression of the hypoxia responsive genes, indicating that these tumors were less hypoxic compared to tumors in the ESCC group. The minimal inter-tumor variability of gene expressions in patients with EGJ/GC did not allow further analysis on the prognostic value of the hypoxia gene expression profile in this subset of patients.
Figure 1. Cluster analysis of the 15 hypoxia-induced genes. (A) Unsupervised cluster analysis of 89 patients. Red colour corresponds to a high gene expression level and green colour corresponds to a low gene expression level. (B) Supervised cluster analysis of patients with ESCC according to ranking (n = 51).
Correlation of hypoxia responsive genes and outcome in ESCC
In the ESCC group 51 patients were ranked according to gene expression of the 15 hypoxia-induced genes, thus, patients ranked low represented more up-regulated gene expression and patients ranked high represented lower gene expression (). Based on the hypoxia gene expression data patients were divided into tertiles. The upper tertile represented more up-regulated gene expression indicating a more hypoxic genotype and the middle and lower tertile represented patients with intermediate and low hypoxic genotypes, respectively. Among the 51 patients analyzed, 47 patients were diagnosed with SCC and four patients with undifferentiated carcinoma. These four patients were characterized as being less hypoxic. Patients in the upper tertile suffered a poorer outcome in terms of OS than patients in the middle and lower tertile [HR = 0.48 (CI 0.21–1.07), p = 0.07] (). Similarly, when evaluating DSS a trend towards worse outcome in the upper tertile was observed compared to the other two tertiles [HR = 0.48 (CI 0.18–1.24), p = 0.13] (). Multivariate Cox proportional hazard regression did not identify a significant association between hypoxia responsive gene expressions and survival () nor did a χ2-test on the correlation between the hypoxic status and treatment response in the ESCC group.
Figure 2. (A) Kaplan-Meier estimates of overall survival and (B) disease-specific survival among patients with a more hypoxic genotype and a less hypoxic genotype based on the 15 hypoxia-induced genes.
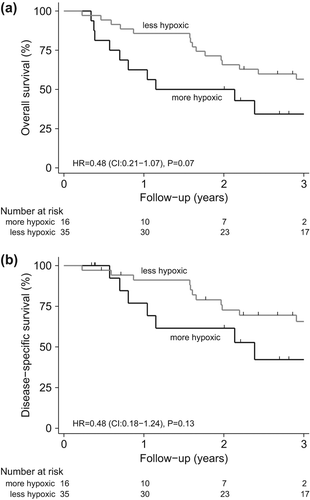
Table II. Possible prognostic factors and relative risk.
Clinicopathological parameters as prognostic factors
Potential clinicopathological prognostic factors, including age, gender, tumor characteristics and treatment response are evaluated in . A follow-up period of 36 months for overall survival and disease-specific survival is illustrated for both the ESCC group and the EGJ/GC group. Univariate analysis of patients in the ESCC group indicated that treatment response was associated with disease-specific survival [HR = 0.17 (CI 0.04–0.73), p = 0.02] but not overall survival (Supplementary Figure 1, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.818247 and ). Similarly, multivariate Cox proportional hazard regression indicated that treatment response was an independent prognostic factor and significantly associated with disease-specific survival [HR = 0.21 (CI 0.05–0.95), p = 0.04] in the ESCC group. In the EGJ/GC group age was significant in a multivariate analysis on both overall survival [HR = 7.94 (CI 1.89–33.38), p = 0.01] and disease-specific survival [HR = 4.79 (CI 1.09–21.09), p = 0.04]. All other covariates did not show any difference, neither for OS nor DSS. Thus, non-complete treatment response and advanced age are associated with a poor survival.
Discussion
This is the first study to evaluate the hypoxic impact of a prognostic and predictive set of genes on survival and treatment response in gastroesophageal cancer. The reason for applying the 15 hypoxia-induced and pH-independent genes developed by Toustrup et al. was that this metagene was developed based on prior knowledge of the hypoxic status of the tumors and had proved to be of prognostic impact as well as predictive beneficial effect of hypoxic modification. In contrast other available hypoxia gene signatures are developed empirically based on the prognostic value [Citation18–20].
In the present study a cluster analysis successfully divided ESCC patients into two clusters of either a more or a less hypoxic genotype. In contrast, all patients in the EGJ/GC group were classified as being less hypoxic and the homogenous gene expression did not allow for discrimination between patients. As expression of hypoxia-responsive genes varied substantially in the ESCC group subsequent data analysis on the prognostic impact of the hypoxia regulated genes was performed in this group only.
The 15 hypoxia gene expression signature along with the previously mentioned hypoxia metagenes were primarily developed in squamous cell carcinomas, but have been shown to be of prognostic significance in non-squamous cell carcinomas (breast and lung cancer) [Citation24,Citation25]. Despite different developmental approaches the signatures have shown overlap of a subset of genes with NDRG1, ALDOA and SLC2A1 being the most frequently represented, involved in the stress response and glucose metabolism. The overlapping of genes indicates a rational of the use of gene expression to identify global hypoxic markers irrespective of the underlying approach. Thus, the diverse classification between ESCC and EGJ/GC (adenocarcinomas) tumors might indicate that in gastroesophageal cancer hypoxia mainly plays a role in squamous cell carcinomas. This, however, needs further research to clarify, not only for gastroesophageal adenocarcinomas but for a range of other histologically distinct tumor types.
The data on patients in the ESCC group showed a trend towards a poorer outcome in terms of OS and DSS for tumors ranking as the most hypoxic third. There was no statistical significant association between this subgroup and survival, but the results indicated that this tertile represented a poor-prognosis group which is consistent with hypoxia being an adverse clinical prognostic factor in various cancers. Similarly, there was no statistical significant association between the gene expression and the treatment response to chemoradiotherapy. The study population was small and in order to determine the real magnitude of the poorer outcome seen in patients with a more hypoxic genotype a larger study population is needed.
As mentioned above other hypoxia gene expression signatures have proven to be of prognostic impact in HNSCC, breast, lung and cervical cancer. In a microarray study of 59 HNSCC Winter et al. obtained a hypoxia signature by analysis of genes that by in vivo expression clustered with the expression of 10 well-known hypoxia-regulated genes. This 99-metagene proved to be an independent prognostic factor for treatment outcome when validated in independent data sets of head and neck cancer (n = 60) and breast cancer (BC) (n = 295) [Citation19]. In a further study by Buffa et al. a common hypoxia profile was defined from three head and neck and five breast cancer studies. The metagene was generated on the basis of co-expression networks of acknowledged hypoxia-associated genes and was reduced from 99 genes to a signature of 51 genes. The prognostic relevance was validated in four independent data sets of lung (n = 216), breast (n = 295) and head and neck cancer (n = 60) and the common hypoxia metagene was more prognostic than several previously reported signatures [Citation18]. By integrating dynamic contrast-enhanced MR imaging (DCE-MRI) and global gene expression data of cervical cancer Halle et al. generated a 31-gene hypoxia profile proven to be of prognostic impact in an independent validation cohort of 109 patients with cervical cancer. This result indicates the use of a non-invasively imaging technique to identify patients with hypoxia-related resistance to chemoradiotherapy [Citation20].
A few studies have addressed the impact of hypoxia in gastroesophageal cancer. Matsuyama et al. evaluated the implications of hypoxia-inducible factor 1α protein expression in 215 resected ESCC specimens. The transcription factor HIF1A is known to be highly involved in the hypoxic response promoting tumor growth, metastasis and angiogenesis and has been shown to be inversely correlated with survival in various cancers [Citation9]. In the study a significant association between HIF-1α protein and disease-free survival, but not OS, was observed [Citation26]. Another study by Tanaka et al. examined carbonic anhydrase 9 (CA9) protein expression, known to be induced under hypoxic conditions resulting in a negative impact on patient survival for various tumor entities, in 127 surgical specimens from ESCC patients [Citation27]. CA9 was significantly correlated with a poor cancer-specific survival and a malignant phenotype in these patients. However, CA9 expression was not identified as an independent prognostic factor in multivariate survival analysis.
Whether single factors such as HIF-1α and CA9 are capable of describing the influence of hypoxia adequately and, thus, function as suitable hypoxic and prognostic biomarkers in gastroesophageal cancer is unknown. It has been suggested that the cumulative information of multiple hypoxia-dependent genes carries out more information on hypoxia than measuring only single gene factors [Citation28]. Further studies are needed to elucidate the prognostic impact of hypoxia in ESCC.
Evaluation of clinicopathological parameters as potential prognostic factors was performed in 56 patients with ESCC and 39 patients with EGJ/GC. There were no major differences in the clinical parameters in terms of age and stage between the present study and previous reports [Citation29,Citation30]. The results of univariate and multivariate analysis by Cox proportional-hazards models showed treatment response to be an independent prognostic factor and significantly associated with disease-specific survival in the ESCC group. Complete remission of cancerous disease is a strong prognostic factor and the role of treatment response in ESCC has been demonstrated in previous studies [Citation31,Citation32]. In the EGJ/GC group age was shown to be an independent prognostic factor in a multivariate analysis on both overall survival and disease-specific survival. The survival worsened in patients with advanced age at diagnosis (age ≥ 62 years).
In the ESCC group, R0 resection was performed in 93% of patients which is consistent with former studies on preoperative chemoradiotherapy with a mean R0 resection rate at 81.7% [Citation31]. Similarly, the observed percentage of patients with complete remission of cancerous disease was in line with previous studies (37% vs. 25.9%) [Citation31].
In conclusion, the 15 hypoxia responsive genes successfully determined the hypoxic genotypes of patients with ESCC and EGJ/GC. However, only patients with ESCC showed heterogeneous gene expression whereas all patients with EGJ/GC were classified as being less hypoxic, indicating that the hypoxic impact in squamous cell carcinomas is more profound. The study showed a trend towards a poorer outcome in terms of OS and DSS among ESCC patients with a more hypoxic genotype. However, this finding is preliminary and future studies are needed to validate these results.
Supplementary Figure 1
Download PDF (192.3 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The study is supported by The Danish Cancer Society, CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research, The A.P. Møller Foundation for the Advancement of Medical Science, Karen A. Tolstrups Fund and The Family Spogaards Fund.
References
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–12.
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009;374:477–90.
- Vallbohmer D, Brabender J, Metzger R, Holscher AH. Genetics in the pathogenesis of esophageal cancer: Possible predictive and prognostic factors. J Gastrointest Surg 2010; 14(Suppl 1):S75–80.
- Wu X, Hedman H, Bergqvist M, Bergstrom S, Henriksson R, Gullbo J, et al. Expression of EGFR and LRIG proteins in oesophageal carcinoma with emphasis on patient survival and cellular chemosensitivity. Acta Oncol 2012;51:69–76.
- Pennathur A, Xi L, Litle VR, Gooding WE, Krasinskas A, Landreneau RJ, et al. Gene expression profiles in esophageal adenocarcinoma predict survival after resection. J Thorac Cardiovasc Surg 2013;145:505–12; discussion 512–3.
- Stiekema J, Boot H, Aleman BM, Wessels LF, van Sandick JW. Prognostication and prediction using gene expression profiling in oesophageal cancer. Eur J Surg Oncol 2013;39:17–23.
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38–47.
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005; 77:18–24.
- Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26:225–39.
- Harrison L, Blackwell K. Hypoxia and anemia: Factors in decreased sensitivity to radiation therapy and chemotherapy?Oncologist 2004;9(Suppl 5):31–40.
- Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev 2007;26: 225–39.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011;100: 22–32.
- Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother Oncol 2012;105:14–20.
- Zips D, Zophel K, Abolmaali N, Perrin R, Abramyuk A, Haase R, et al. Exploratory prospective trial of hypoxia- specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 2012;105:21–8.
- Tran LB, Bol A, Labar D, Jordan B, Magat J, Mignion L, et al. Hypoxia imaging with the nitroimidazole 18F-FAZA PET tracer: A comparison with OxyLite, EPR oximetry and 19F-MRI relaxometry. Radiother Oncol 2012;105: 29–35.
- Mortensen LS, Busk M, Nordsmark M, Jakobsen S, Theil J, Overgaard J, et al. Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: A preclinical study. Radiother Oncol 2011;99:418–23.
- Shen Z, Kauttu T, Seppanen H, Vainionpaa S, Ye Y, Wang S, et al. Both macrophages and hypoxia play critical role in regulating invasion of gastric cancer in vitro. Acta Oncol 2013;52:852–60.
- Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer 2010; 102:428–35.
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res 2007;67:3441–9.
- Halle C, Andersen E, Lando M, Aarnes EK, Hasvold G, Holden M, et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res 2012;72:5285–95.
- Toustrup K, Sorensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 2011; 71:5923–31.
- Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012;102:122–9.
- Sorensen BS, Toustrup K, Horsman MR, Overgaard J, Alsner J. Identifying pH independent hypoxia induced genes in human squamous cell carcinomas in vitro. Acta Oncol 2010;49:895–905.
- Toustrup K, Sorensen BS, Alsner J, Overgaard J. Hypoxia gene expression signatures as prognostic and predictive markers in head and neck radiotherapy. Semin Radiat Oncol 2012;22:119–27.
- Starmans MH, Chu KC, Haider S, Nguyen F, Seigneuric R, Magagnin MG, et al. The prognostic value of temporal in vitro and in vivo derived hypoxia gene-expression signatures in breast cancer. Radiother Oncol 2012;102:436–43.
- Matsuyama T, Nakanishi K, Hayashi T, Yoshizumi Y, Aiko S, Sugiura Y, et al. Expression of hypoxia-inducible factor- 1alpha in esophageal squamous cell carcinoma. Cancer Sci 2005;96:176–82.
- Tanaka N, Kato H, Inose T, Kimura H, Faried A, Sohda M, et al. Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer 2008; 99:1468–75.
- Moon EJ, Brizel DM, Chi JT, Dewhirst MW. The potential role of intrinsic hypoxia markers as prognostic variables in cancer. Antioxid Redox Signal 2007;9:1237–94.
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol 2007;8:226–34.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
- Courrech Staal EF, Aleman BM, Boot H, van Velthuysen ML, van Tinteren H, van Sandick JW. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg 2010;97:1482–96.
- Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 2005;6:659–68.
