Abstract
Background. Tumour volume change during delivery of chemoradiotherapy is observed in small cell lung cancer (SCLC) patients. In this study, we have compared tumour volume and anatomical changes, e.g. atelectasis or pleural effusions determined by three different methods. Method. A total of 37 SCLC patients undergoing thoracic radiotherapy during 2010–2011 were included. The patients were treated based on a daily three-dimensional (3D) cone beam computed tomography (CBCT) bony anatomy registration. The CBCT scans were retrospectively reviewed visually by a radiation therapist (Visual-RTT) in order to register tumour volume changes. Furthermore, the tumour volume changes were obtained by either deformable image registration (DIR) or delineation by a radiation oncologist (RO). Kappa (κ) statistics and paired t-tests were used for evaluation of the inter-tester agreement. Results. The tumour volume change between the Visual-RTT, the DIR and the RO assessments obtained 84–97% agreement (κ = 0.68–0.95). Furthermore, there was no statistically significant difference between the tumour change assessment of the RO (mean 13.6 ml) and the DIR (mean 14.5 ml), p = 0.59. Tumour shrinkage was observed in 15 (41%) patients and anatomical changes in seven (19%) patients. Conclusion. The inter-tester reproducibility of tumour volume change between the three methods is excellent. Visual-RTT on-line inspection may be used to determine tumour shrinkage and anatomical changes as atelectasis or pleural effusions during the radiotherapy course by use of daily CBCT scans.
Small cell lung cancer (SCLC) is a disease considerably influenced by tumour changes during delivery of chemoradiotherapy [Citation1]. Significant lung tumour shrinkage during the radiotherapy (RT) course has been observed and the pattern of tumour shrinkage was very heterogeneous [Citation2,Citation3]. In some patients, a large tumour volume change was observed and in others there was no change during an entire RT course [Citation4]. In a previous study from our institution, we observed a significant shrinkage (15–40%) of the gross tumour volume of the primary tumour (GTV-T) from planning computed tomography (pCT) until the last cone beam (CB) CT in 40% of the patients [Citation5]. These studies investigated the tumour volume change off-line by delineation of the GTV by a radiation oncologist (RO).
The development of image-guided (IG) RT has enabled a visual, on-line identification of tumour volume change, e.g. shrinkage on a daily basis. Moreover, anatomical changes related to density variations in the lung tissue, e.g. changes in atelectasis or pleural effusion may be observed. Such changes may lead to deviations in the position of the tumour compared to the pCT and thus dosimetrical changes may appear [Citation6–11]. Tumour shrinkage and lung tissue changes can be accounted for by using an adaptive approach for RT of SCLC to minimise the dose to normal structures, allowing for dose escalation and/or reduction of treatment morbidity [Citation12,Citation13].
Adaptive RT introduces a huge workload on the clinic as it requires repeated CT scan, target delineations and treatment re-planning. Automated target delineation on the repeated CT scans may therefore reduce the workload. However, deviations between delineations performed by the RO and the deformable image registration (DIR) method have been observed [Citation14]. Therefore, automated structure propagation by DIR has to be validated.
The aim of this study was to investigate the possibility of online visual identification of tumour volume and anatomical changes during a RT course using the daily CBCT scans for the treatment of SCLC patients. Furthermore, a comparison of the inter-tester agreement on the determination of tumour volume change between the visually radiation therapist (Visual-RTT) assessment, the delineation by the RO and the DIR method was made.
Material and methods
Patients
A total of 37 SCLC cancer patients were treated with radical chemoradiotherapy and prophylactic cranial irradiation (PCI) [Citation15]; 25 Gy in 10 fractions (Fr), 5 Fr/week, between January 2010 and December 2011. Thirty-one (84%) patients were treated in the thoracic region with 45 Gy in 30 Fr, 10 Fr/week and six (16%) patients were treated with 50 Gy in 25 Fr, 5 Fr/week. Most patients (89%) were treated with 4–6 cycles of cisplatin or carboplatin and etoposide concomitant with RT. The median follow-up time was 25 months (range 4–40 months) and 29 months (range 17–40 months) for patients still alive at the time of analysis.
Treatment planning
The patients underwent a free breathing four- dimensional (4D) scanning in the supine position on a narrow cone beam helical CT scanner (Philips LB16, Philips Healthcare, Best, The Netherlands). The patients were fixed with both arms above the head in an individual immobilisation device. The isocentre was marked on the patients with three skin tattoos and the scan range covered the complete thoracic region. Intravenous contrast was supplied before the scan. The median time interval from the pCT until start of RT was 11 days (range 7–21). The tumour and the normal tissue structures were delineated at the pCT using the mid ventilation phase defined as the mean bin in the respiration cycle in the Eclipse treatment planning system (VMS, Palo Alto, CA, USA). A 3D conformal or intensity- modulated treatment plan using three to eight 6MV beams was created.
Patient setup and in-room imaging
For every treatment session, the patients were aligned according to the skin tattoos by use of the in-room laser system. Thereafter, a kV CBCT scan [Citation16] was acquired with the gantry-mounted onboard imager (OBI) (VMS) [Citation17]. The CBCT scan was rigidly registered to the pCT scan. An automatic registration of the bony anatomy was performed using a user defined region of interest including the spine. The registration was evaluated by the RTTs and the resulting translational shifts in three dimensions were effectuated.
Retrospective tumour volume determination – radiation oncologist (RO)
A senior RO delineated GTV-T at the CBCT scan of the last fraction of radiotherapy for all 37 patients. The delineation of the tumour at the pCT scan was used as guidance. The tumour volume was measured at the pCT and the CBCT scan after the delineation. The volumetric change was determined as an absolute value of the pCT volume for each patient. Absolute changes ≥ 5 cm3 for small tumours or relative changes ≥ 15% for large tumours were considered as tumour shrinkage or growth. The RO used the lung window (−1000 HU–−200 HU) when contouring tumour in lung tissue, whereas the mediastinal window (−15 HU–85 HU) was used when the tumour was located close to the mediastinum or thoracic wall.
Retrospective tumour volume determination – deformable image registration (DIR)
The pCT was used as reference image for a registration of the pCT image to each of the CBCT images acquired at fraction number 1, 6, 11, 16, 21, 26(25) and 30. First, a rigid registration based on bony anatomy was made. Second, a demons-based DIR was performed using Smart Adapt (VMS). Finally, the GTV-T was propagated from pCT to each of the selected CBCT images using the DIR. The tumour volume was measured at the selected CBCT scans. The DIR algorithm was demons-based and was expected to be inaccurate when large deformations were present, e.g. disappearance of an atelectasis. Therefore, all deformations were visually inspected and corrected if necessary.
The volume of GTV-T (VDIR) obtained by DIR was compared with the volume of GTV-T delineated by the RO (VRO) by calculation of the Dice similarity coefficient (DSC) [Citation18]:
Retrospective tumour volume determination – visual-radiation therapist (Visual-RTT)
The CBCT scans of each fifth fraction were selected for visual inspection by only one RTT in an off-line imaging system (Offline Review, VMS). The GTV-T was visible at the CBCT scans for all patients. No contouring was made at the CBCT scans for this evaluation. For all selected scans, the size of the primary tumour at the CBCT scan was visually compared to the size of GTV-T delineated at the pCT and to the size of the GTV-T at the CBCT acquired five fractions in advance of the actual CBCT. Tumour shrinkage or growth was scored for each selected fraction.
Furthermore, the CBCT scans were visually inspected in order to observe major anatomical changes related to an appearing or disappearing atelectasis or pleural effusion.
Statistical analysis
The statistical analysis was performed using STATA version 12.0. Kappa (κ) statistics [Citation19] was used to assess reproducibility of tumour volume change between the three methods: Visual-RTT versus DIR or RO assessments. The RO assessments were used as the golden standard to assess the validity of the Visual-RTT and DIR method. The answer to the test was that the patient either has reached a tumour volume change (positive test) or not (negative test) and the cut-off for the κ value was set at 60% [Citation19]. We used the Bland Altman plot and paired t-tests for interval data to analyse the agreement in the tumour volume change between the DIR and the RO assessments.
The survival fraction and local relapse were calculated from the time of diagnosis. Local relapse was defined as recurrence in the radiation field by RO assessments. Patients were censored from the date of last follow-up. The Cox proportional hazard method related to survival and the Fisher Exact-test related to local relapse were used for comparison of local recurrence/death and tumour volume change as obtained from the RO assessments.
Results
RO, DIR and Visual-RTT assessments of the tumour volume change
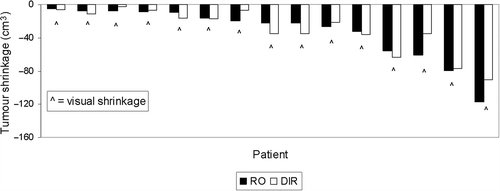
At the last treatment fraction, tumour shrinkage ≥ 5 cm3 or 15% was observed in 15 patients (41%) by the RO and the mean decrease in the initial tumour volume was 27%. Comparison to the DIR and to the Visual-RTT evaluation showed tumour shrinkage in the same 15 patients (). No change was observed in 22 patients, as determined by the RO. Of these DIR showed tumour shrinkage in three patients and growth in one patient and the Visual-RTT showed tumour shrinkage in three patients.
Inter-tester reproducibility
The agreement of the scoring of tumour volume change between the Visual-RTT and the DIR evaluation at every fifth fraction is shown in . All observed agreements were within 84–97%, (κ, 68–95%) with prevalences in the range of 45–54%. At the last treatment fraction the Visual-RTT, DIR and RO assessments had an overall agreement of 84%.
Table I. Inter-tester reproducibility of tumour change between deformable image registration (DIR) and visual radiation therapist (Visual-RTT) assessment for every 5th fraction of radiotherapy.
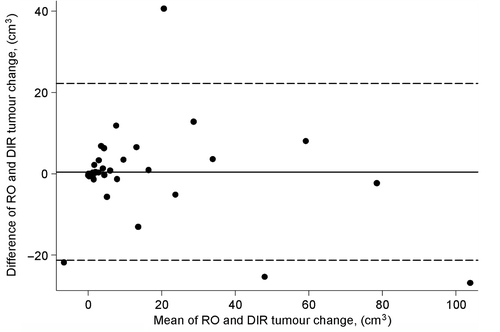
The mean tumour volume change as determined by RO and DIR was compared to the difference in tumour volume change at the last treatment fraction (). The mean difference between the DIR and the RO was 1.0 cm3 [95% confidence interval (CI): −2.6; 4.6 cm3]. Four observations (8%) fell outside the 95% limits of agreement. There was no significant difference between the tumour volume change assessments by the RO (mean: 13.6cm3; −4.3; 117.3) and DIR (mean: 14.5 cm3; −17.5; 90.4), p = 0.59.
Figure 2. Bland Altman plot of the mean compared to the difference in tumour volume change obtained by the DIR and RO with 95% limits of agreement (broken lines).
The mean DICE similarity coefficient was 0.82 (range 0.65–0.98) with the lowest value observed for a patient with a large anatomical change related to disappearance of an atelectasis.
Pattern of survival, tumour recurrence and retrospective Visual-RTT observation of major anatomical changes
Of the 37 patients, five (13%) had a local relapse of the disease and 17 (46%) had died. There was no statistically significant difference in local recurrence (p = 0.38) and in overall survival (p = 0.94) related to tumour volume change above 5 cm3 or 15%.
Seven (19%) patients had major anatomical changes of the lung tissue. Four patients (11%) had an atelectasis at the pCT scan. The atelectasis disappeared before fraction number 16. In two patients (5%) a pleura effusions varied during the treatment course. One patient (3%) had both pleura effusion and atelectasis at the pCT scan.
Discussion
The present study showed excellent agreement between three different methods of determining tumour volume changes during RT. On-line evaluation of tumour volume changes or lung tissue changes makes it possible to set up a strategy for adaptive treatment planning for SCLC patients using the daily CBCT scans [Citation13].
In the present study, tumour shrinkage or growth was defined as a volumetric change of more than 5 cm3 for small tumours or 15% for large tumours. This was based on a Visual-RTT evaluation and only changes of this order of magnitude were evaluable. Tumour shrinkage (seen in 41% of the patients) and anatomical changes as varying amounts of atelectasis or pleural effusions (in 19% of the patients) were visually identified by the RTT in this study. Similar findings were obtained in other studies, which relies on delineation of the tumour at CT or CBCT images obtained during the treatment course [Citation2–5]. The results from Juhler-Nottrup et al. [Citation2] and Fox et al. [Citation4] were difficult to compare with due to different imaging schedules; the use of megavoltage CT imaging, which may not provide the same quality of image resolution of thoracic tumours as kilovoltage CT; and the non-uniform use of concurrent chemotherapy. Hugo et al. showed that lung tumour regression during radiotherapy can introduce geometrical changes in the normal tissue surrounding the tumour that affect the tumour volume, shape and position [Citation20].
We found no significant difference between the tumour volume change assessments by the RO and the DIR method. The mean DSC for the two methods was 0.82 (range 0.65–0.98), which is a decent agreement. In a recent study, DIR and contour propagation to the 10 respiratory phases of 4DCT scans was investigated for 10 lung cancer patients [Citation14]. Manual delineation and delineation based on automated propagation of the tumour were compared and no significant difference was found. Ezhil et al. [Citation21] compared three different methods of propagating the tumour to the 10 respiratory phases of 4DCT scans. They found a better agreement for rigid propagation of the tumour than for DIR propagated tumours. This was primarily due to a reduction in the tumour volume when DIR was used. Such a volumetric reduction was not seen in the present study. A validation of different algorithms for DIR using virtual deformable phantoms showed gross errors for thoracic structures when using demons-based algorithms, whereas B-spline algorithms showed better agreement [Citation22]. The deviations were primarily ascribed to different contrast in the images and large deviations were seen especially for the heart, whereas the lung tumour showed a DSC of 0.83. In a recent study from our institution, Nyeng et al. [Citation23] investigated a former version of the demons-based algorithm in Smart Adapt. A comparison of DIR of the inhale and the exhale respiratory phases of 4DCT scans of five patients and DIR of the exhale and the inhale phases (reversed order) showed deviations of 3–6 mm even though ideally, no deviation should be obtained. Similar deviations (mean 3 mm ± 2 mm) were observed in a study by Yim et al. [Citation24] investigating another demons-based algorithm. Speight et al. [Citation25] found a mean DSC of 0.86 and a mean distance to agreement of 1 mm for both a B-spline and a demons-based algorithm in a study of 25 lung cancer patients. This is in concordance with the present study.
We acknowledge that the lower contrast in the CBCT scans compared to the CT scans makes the delineations of the GTV-T less accurate. However, as the tumour volume changes should be evaluated on-line on a daily basis from the CBCT scans, we will have to compare the visual assessment to the RO off-line assessment using the CBCT scans.
The Visual-RTT assessed tumour volume change was based on subjective assessments, which could lead to different inter-tester assessments related to a learning curve effect. However, we used the RO assessment of the tumour volume as the quantitative golden standard and the κ values between the Visual-RTT and RO or DIR assessments were 68–95% proposing a nice agreement of the methods. Siker et al. [Citation26] reported greater inter-observer variability in tumours abutting the mediastinum or with atelectasis on megavoltage CT.
The survival data was comparable with other retrospective consecutive studies of patients [Citation27–29]. We found no significant difference in survival and local recurrence related to changes in tumour volume, which was expected due to the low number of patients.
We propose that the RTTs should be trained in recognising tumour volume and lung density changes. Furthermore, a decision algorithm should be set up in order to categorise these visible changes and apply the necessary actions to be taken. The implementation of such a decision algorithm will require close collaboration between RTTs, RO and medical physicists resulting in more accurate treatment of the target volume. The DIR may be used to propagate the target to the rescanning CT followed by a revision of the structures a RO and an adaptive treatment plan in order to deliver an optimal dose distribution [Citation30].
In conclusion, this study has demonstrated that Visual-RTT on-line inspection may be used to determine tumour shrinkage and anatomical changes as effusion or atelectasis during the radiotherapy course by use of daily CBCT scans. Furthermore, the inter-tester reproducibility of tumour change between the Visual-RTT, DIR and RO assessments was excellent.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supported by CIRRO – The Lundbeck Foundation Centre for Interventional Research in Radiation Oncology.
References
- Jassem J. The role of radiotherapy in lung cancer: Where is the evidence?. Radiother Oncol 2007;83:203–13.
- Juhler-Nottrup T, Korreman SS, Pedersen AN, Persson GF, Aarup LR, Nystrom H, et al. Interfractional changes in tumour volume and position during entire radiotherapy courses for lung cancer with respiratory gating and image guidance. Acta Oncol 2008;47:1406–13.
- Yee D, Rathee S, Robinson D, Murray B. Temporal lung tumor volume changes in small-cell lung cancer patients undergoing chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;80:142–7.
- Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;74:341–8.
- Knap MM, Hoffmann L, Nordsmark M, Vestergaard A. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol 2010;49:1077–84.
- Britton KR, Starkschall G, Liu H, Chang JY, Bilton S, Ezhil M, et al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;73:94–102.
- Bissonnette JP, Purdie TG, Higgins JA, Li W, Bezjak A. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys 2009; 73:927–34.
- Yamashita H, Haga A, Hayakawa Y, Okuma K, Yoda K, Okano Y, et al. Patient set up error and day-to-day esophageal motion error analyzed by cone-beam computed tomography in radiation therapy. Acta Oncol 2010;49:485–90.
- Nielsen M, Bertelsen A, Westberg J, Jensen HR, Brink C. Cone beam CT evaluation of patient set up accuracy as a QA tool. Acta Oncol 2009;48:271–6.
- Waldeland E, Ramberg C, Arnesen MR, Helland A, Brustugun OT, Malinen E. Dosimetric impact of a frame-based strategy in stereotactic radiotherapy of lung tumors. Acta Oncol 2012;51:603–9.
- Josipovic M, Persson GF, Logadottir A, Smulders B, Westmann G, Bangsgaard JP. Translational and rotational intra- and inter-fractional errors in patient and target position during a short course of frameless stereotactic body radiotherapy. Acta Oncol 2012;51:610–7.
- Møller DS, Khalil AA, Knap MM, Muren LP, Hoffmann L. A planning study of radiotherapy dose escalation of PET-active tumour volumes in non-small cell lung cancer patients. Acta Oncol 2011;50:883–8.
- Sonke JJ, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol 2010;20:94–106.
- Gaede S, Olsthoorn J, Louie AV, Palma D, Yu E, Yaremko B, et al. An evaluation of an automated 4D-CT contour propagation tool to define an internal gross tumour volume for lung cancer radiotherapy. Radiother Oncol 2011;101: 322–8.
- Ramlov A, Tietze A, Khalil AA, Knap MM. Prophylactic cranial irradiation in patients with small cell lung cancer. A retrospective study of recurrence, survival and morbidity. Lung Cancer 2012;77:561–6.
- Pouliot J. Megavoltage imaging, megavoltage cone beam CT and dose- guided radiation therapy. Front Radiat Ther Oncol 2007;40:132–42.
- Sorcini B, Tilikidis A. Clinical application of image-guided radiotherapy, IGRT (on the Varian OBI platform). Cancer Radiother 2006;10:252–7.
- Dice LR. Measures of the amount of ecologic association between species. Ecology 1945;26:297–302.
- Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74.
- Hugo GD, Weiss E, Badawi A, Orton M. Localization accuracy of the clinical target volume during image-guided radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2011;81:560–7.
- Ezhil M, Choi B, Starkschall G, Bucci MK, Vedam S, Balter P. Comparison of rigid and adaptive methods of propagating gross tumor volume through respiratory phases of four-dimensional computed tomography image data set. Int J Radiat Oncol Biol Phys 2008;71:290–6.
- Varadhan R, Karangelis G, Krishnan K, Hui SJ. A framework for deformable image registration validation in radiotherapy clinical applications. Appl Clin Med Phys 2013;14:4066–76.
- Nyeng TB, Kallehauge JF, Højer M, Petersen JB, Poulsen PR, Muren LP. Clinical validation of a 4D-CT based method for lung ventilation measurement in phantoms and patients. Acta Oncol 2011;50:897–907.
- Yim Y, Hong H, Shin YG. Deformable lung registration between exhale and inhale CT scans using active cells in a combined gradient force approach. Med Phys 2010;37: 4307–17.
- Speight R, Sykes J, Lindsay R, Franks K, Thwaites D. The evaluation of a deformable image registration segmentation technique for semi-automating internal target volume (ITV) production from 4DCT images of lung stereotactic body radiotherapy (SBRT) patients. Radiother Oncol 2011;98: 277–83.
- Siker ML, Tomè WA, Mehta MP. Tumor volume changes on serial imaging with megavoltage CT for non-small-cell lung cancer during intensity-modulated radiotherapy: How reliable, consistent, and meaningful is the effect?Int J Radiat Oncol Biol Phys 2006;66:135–41.
- Xia B, Chen GY, Cai XW, Zhao JD, Yang HJ, Fan M, et al. Is involved-field radiotherapy based on CT safe for patients with limited-stage small-cell lung cancer?Radiother Oncol 2012;102:258–62.
- Giuliani ME, Lindsay PE, Sun A, Bezjak A, Le LW, Brade A, et al. Locoregional failures following thoracic irradiation in patients with limited-stage small cell lung carcinoma. Radiother Oncol 2012;102:263–7.
- Yee D, Butts C, Reiman A, Joy A, Smylie M, Fenton D, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 2012;102:234–8.
- Tanyi JA, Fuss MH. Volumetric image-guidance: Does routine usage prompt adaptive re-planning?An institutional review. Acta Oncol 2008;47:1444–53.